Analysis method for determining hydromorphone hydrochloride bulk drug element impurities
A technology for hydromorphone hydrochloride and elemental impurities is applied in the analysis field of determining the elemental impurities of hydromorphone hydrochloride crude drug, can solve problems such as development and research of an elemental impurity analysis method without hydromorphone hydrochloride crude drug, and achieves easy operation, The effect of reducing the acid rush temperature and reducing the signal drift
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] An analytical method for measuring elemental impurities of hydromorphone hydrochloride crude drug, said method comprising the following steps:
[0047] 1. Preparation of diluent (2% nitric acid solution-2% hydrochloric acid solution): accurately measure 1mL of electronically pure grade nitric acid, and set the volume to 500mL with ultrapure water; Fill it into a 500mL volumetric flask [the volumetric flask is made of polyethylene terephthalate (PET), the same below]. Mix the above two solutions 1:1 evenly.
[0048] 2. Preparation of gold standard solution (10μg / mL): Pipette 0.5ml gold single element standard solution (1000μg / mL) [The manufacturer is the National Nonferrous Metals and Electronic Materials Analysis and Testing Center, and the single element standard solutions below are all here Manufacturer] in a 50ml graduated volumetric flask, dilute with ultrapure water and set the volume to the 50ml mark, and shake well.
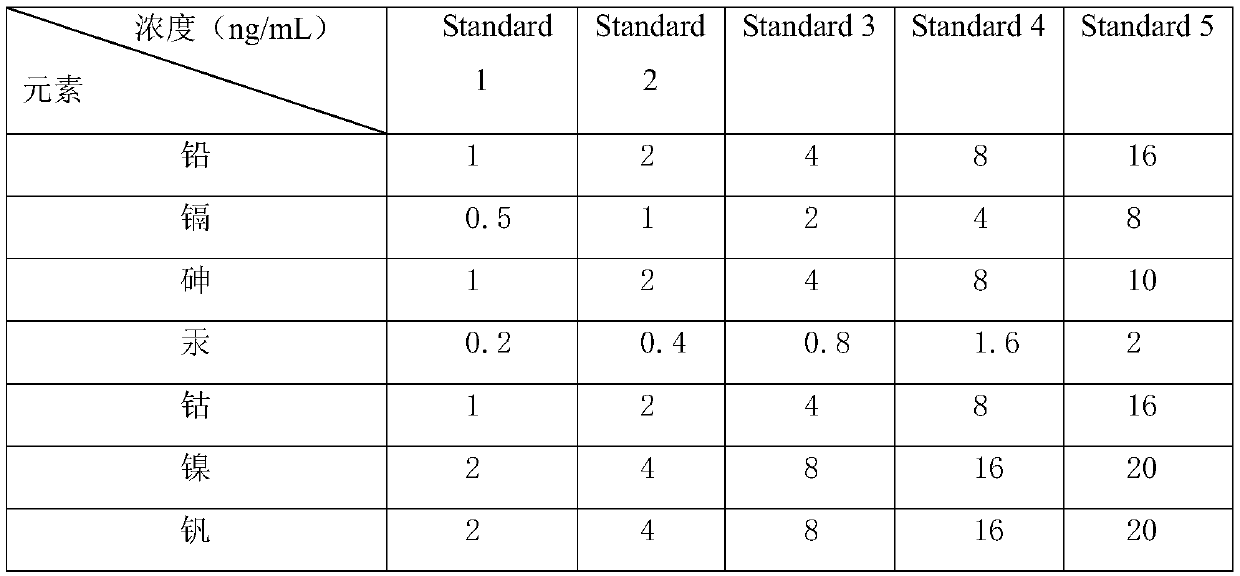
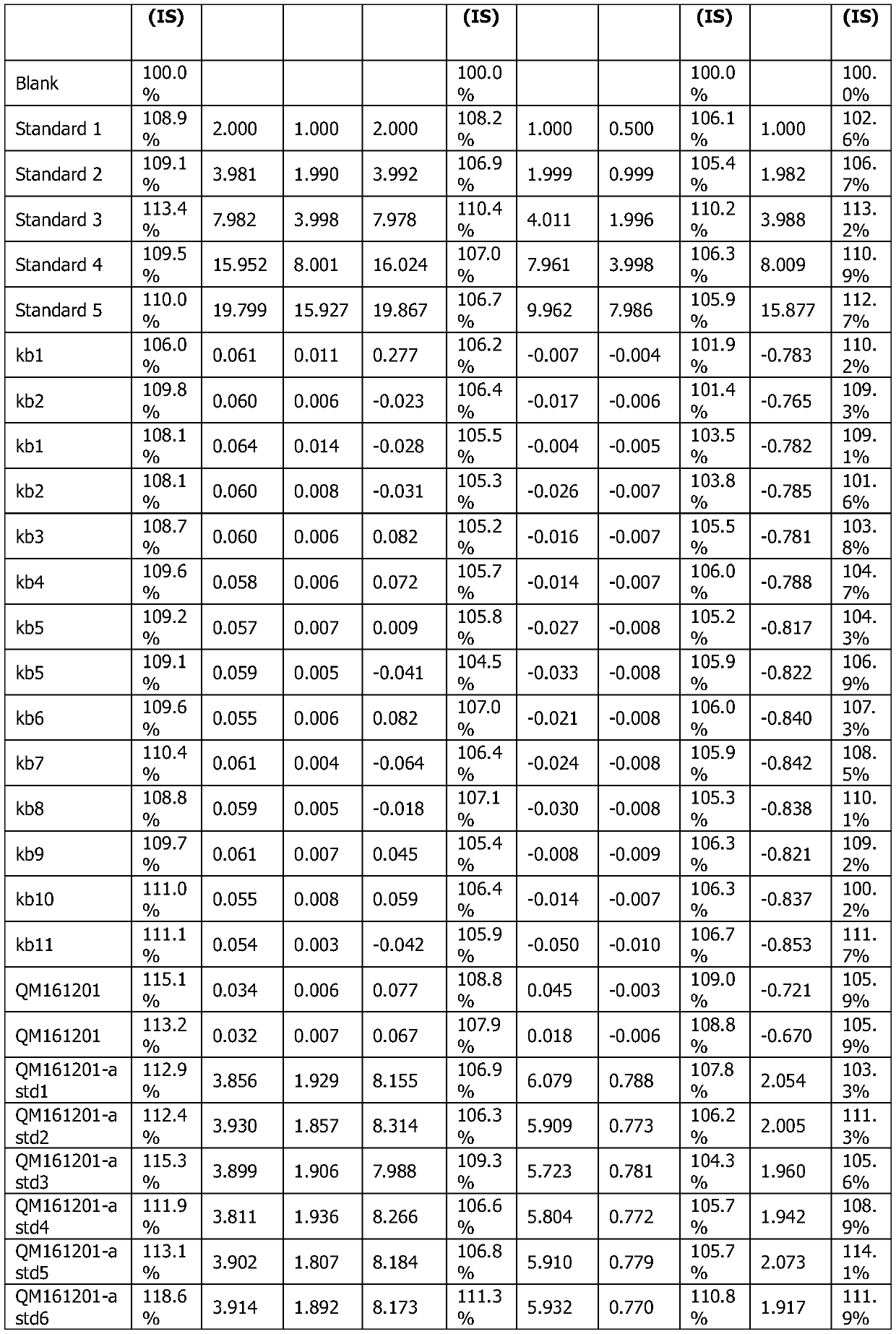
[0049] 3. Preparation of standard solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com