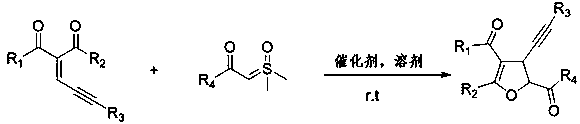
High-effective synthesis of dihydrofuran derivative through Lewis acid catalyzed insertion reaction
A technology of furan derivatives and insertion reaction, applied in the direction of organic chemistry, can solve the problems of lengthy steps, use of toxic organic solvents, poor substrate compatibility, etc., and achieve the effect of simple steps and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0014] Implementation Case 1: Synthesis of Compound 1
[0015]
[0016] Add enynone (38.4 mg, 0.2 mmol), phenylsulfide ylide (98 mg, 0.50 mmol), zinc chloride (1.3 mg, 0.1 mmol) and [BMIM]NTf in a clean reactor successively. 2 Ionic liquid (2 mL), put in a pot at room temperature and stir overnight. After the reaction, it was separated and purified directly by silica gel column chromatography to obtain a yellow oil with a yield of 85%. 1 H NMR (400MHz, CDCl 3 ) δ 8.16 – 8.01 (m, 2H), 7.65 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 8.0Hz, 2H), 5.78 (d, J = 6.0 Hz, 1H), 4.28 (dd, J = 5.6, 1.2 Hz, 1H), 2.32 (d, J = 0.8 Hz, 3H), 2.27 (s, 3H), 1.25 (s, 9H); 13 C NMR (151 MHz, Chloroform-d) δ194.6, 193.0, 168.4, 134.5, 133.8, 129.5, 129.2, 113.0, 92.9, 87.8, 77.9,38.1, 31.3, 29.8, 27.8, 15.2): HRMS ( m / z Calculated value C 20 h 23 o 3 : [M+H + ] 311.1642; found 311.1641.
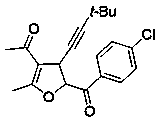
Embodiment example 2
[0017] Implementation Case 2: Synthesis of Compound 2
[0018]
[0019] Add enynone (38.4 mg, 0.2 mmol), 4-methylphenylsulfide ylide (105 mg, 0.50 mmol), zinc chloride (1.3 mg, 0.1 mmol) and [BMIM]NTf to a clean reactor successively. 2 Ionic liquid (2 mL), put in a pot at room temperature and stir overnight.
[0020] After the reaction, it was separated and purified directly by silica gel column chromatography to obtain a yellow oil with a yield of 82%. 1 H NMR (400 MHz, Chloroform- d ) δ 7.98 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 5.76 (d, J = 6.0 Hz, 1H), 4.24 (d, J = 5.6 Hz, 1H), 2.44 (s, 3H), 2.31 (s,3H), 2.26 (s, 3H), 1.24 (s, 9H); 13 C NMR (101 MHz, Chloroform-d) δ 193.3,191.3, 167.1, 144.3, 129.9, 128.5, 128.3, 111.6, 91.4, 86.4, 76.7, 36.8,30.0, 28.5, 26.5, 20.8, 13.9: HRMSI; m / z Calculated value C 21 h 25 o 3 : [M+H + ] 325.1798; found 325.1798.
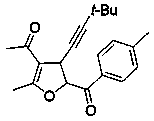
Embodiment example 3
[0021] Implementation Case 3: Synthesis of Compound 3
[0022]
[0023] Add enynone (38.4 mg, 0.2 mmol), 4-methoxyphenylthioylide (113 mg, 0.50 mmol), brominated ketone (2.23 mg, 0.1 mmol), [BMIM] NTf 2 Ionic liquid (2 mL), put in a pot at room temperature and stir overnight. After the reaction, it was directly separated and purified by silica gel column chromatography to obtain a yellow oil with a yield of 69%. 1 HNMR (400 MHz, CDCL 3 ) δ 8.07 (d, J = 8.8 Hz, 2H), 6.98 (d, J = 8.8 Hz, 2H), 5.74 (d, J = 6.0 Hz, 1H), 4.27 (d, J = 6.0 Hz, 1H), 3.90 (s, 3H), 2.31 (s,3H), 2.27 (s, 3H), 1.24 (s, 9H); 13 C NMR (101 MHz, Chloroform- d ) δ 193.3, 190.2, 167.0, 163.4, 130.6, 125.6, 113.1, 111.7, 91.5, 86.4, 76.8, 54.6, 36.8, 30.0, 28.5 26.5, 13.9; HRMS (ESI): m / z Calculated value C 21 h 25 o 4 : [M+H + ] 341.1753; found 341.1752.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com