Preparation method and application of cyanovirin N
An anti-virus and protein technology, applied in the field of genetic engineering, can solve the problem of not getting good soluble expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
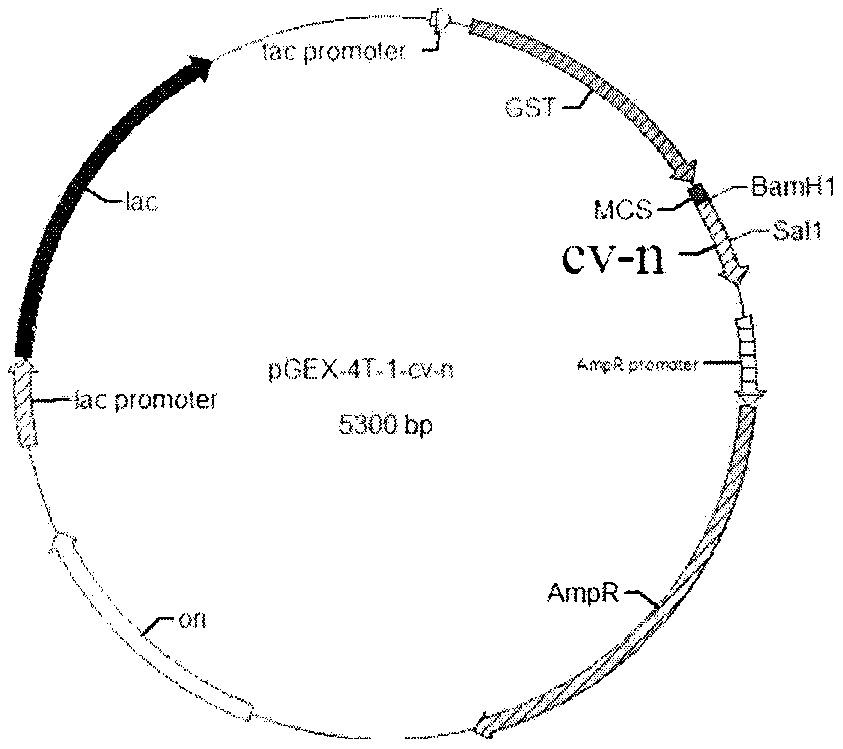
[0037] The construction of embodiment 1 plasmid pGEX-4T-1-cv-n plasmid
[0038] According to the characteristics of the pGEX-4T-1 vector sequence and cv-n sequence, select an appropriate restriction site, and design primers according to the general principles of primer design. Following primers are required primers of the present invention:
[0039]
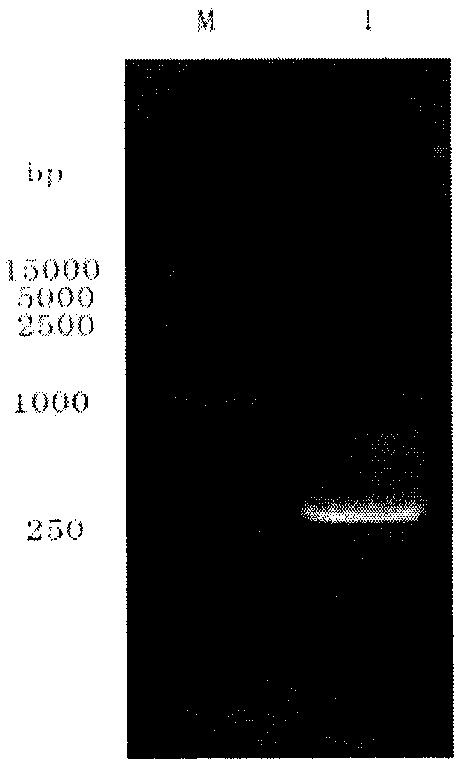
[0040] Amplify the target sequence by PCR. The reaction system is 2 μl of PUC plasmid (about 1ng), 1 μl of cv-n R / cv-n F, 25 μl of PCRMIX, 21 μl of water, and 50 μl in total. Denaturation for 30s, annealing at 50°C for 30s, extension at 72°C for 45s, 30 cycles, and finally 5min at 72°C. The full length of the cv-n gene is 303bp, and the reaction product was identified by 1% agarose gel electrophoresis in line with the expected size ( figure 1 ).
[0041] Gel recovery obtained the full-length cv-n sequence, and the gel recovery product was connected to the T vector. The connection ratio was in accordance with the instruction...
Embodiment 2
[0043] Expression purification identification of embodiment 2 recombinant protein
[0044] 1. Screening and induction of expression strains
[0045] Pick the recombinants with correct sequencing, expand the culture, extract the plasmids and transform them into the expression host OrigamiB (DE3), screen the transformants with LB containing ampicillin and kana, pick a single colony until it contains 50 μg / ml ampicillin, 10 μg / ml In Kanna's 200ml LB liquid medium, expand the culture at 37°C and 220r / min. When the OD value reaches 0.6, take 1ml of the uninduced control, centrifuge to collect the bacterial pellet, add 1*SDS loading buffer, and boil it for later use. For the rest, IPTG was added to make the final concentration 0.1 mol / l. 30°C, 200r / min, induce expression for 3 hours.
[0046] 2. Purification of expression products
[0047] Collect the bacteria by centrifugation, add 3ml of bugbuster protein extraction reagent to resuspend 100ml of bacterial culture, and mix well...
Embodiment 3
[0051] Embodiment 3 chromatographic analysis of cyanobacteria antiviral protein N purity
[0052] The chromatographic column is Sepax SRT SEC-150A (7.8mm×300mm, 5μm), take an appropriate amount of CV-N, dissolve it in PBS to make the final concentration reach 1mg / ml, and use it as the test solution. Use 5mM ammonium acetate:methanol=100:1 solution as the mobile phase for isocratic elution, the flow rate is 0.6ml / min, and the column temperature is 30°C. Absorbance at 280 nm was detected with a DAD detector. Recorded and drawn by computer, by Figure 11 A single elution peak can be seen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com