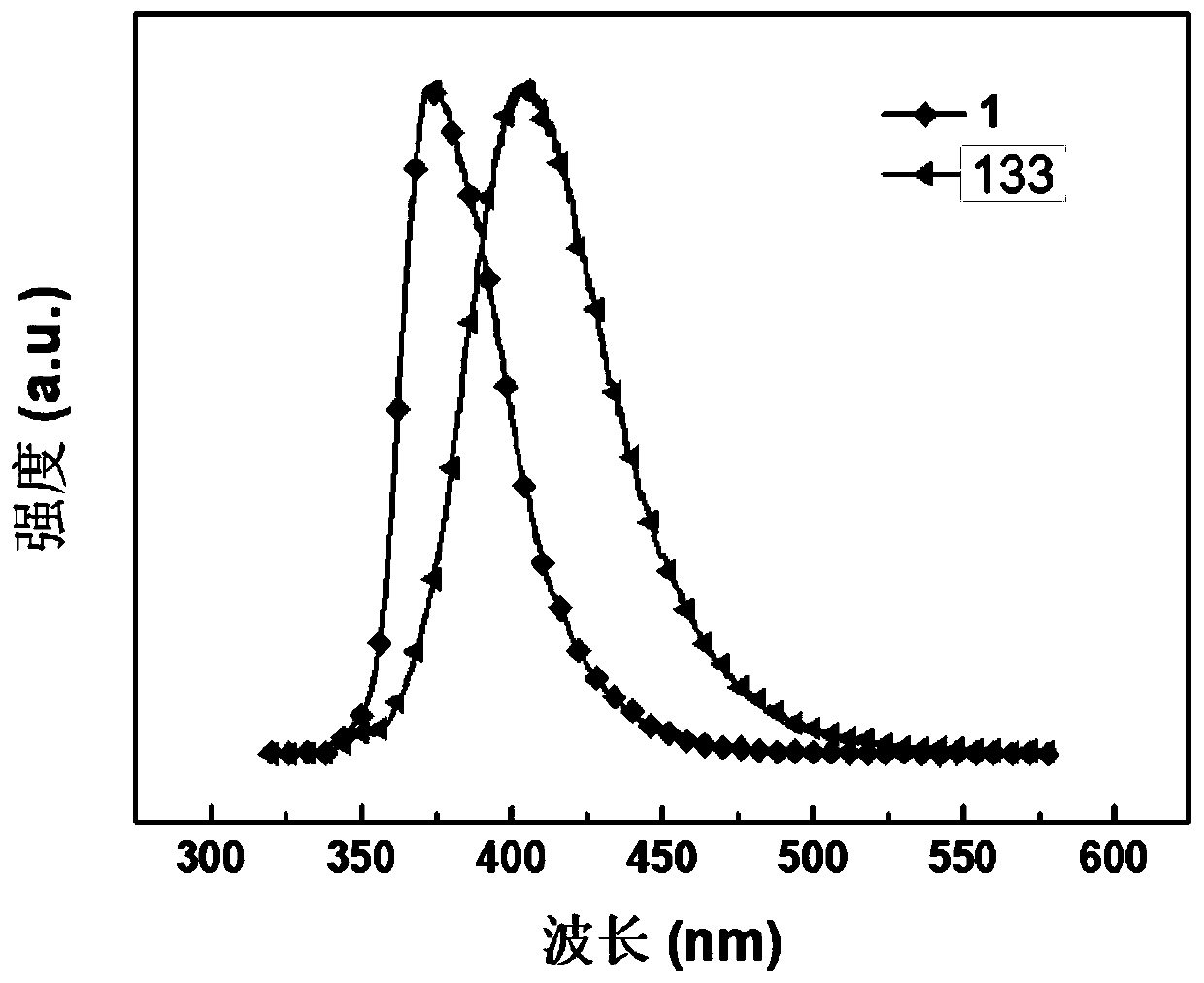
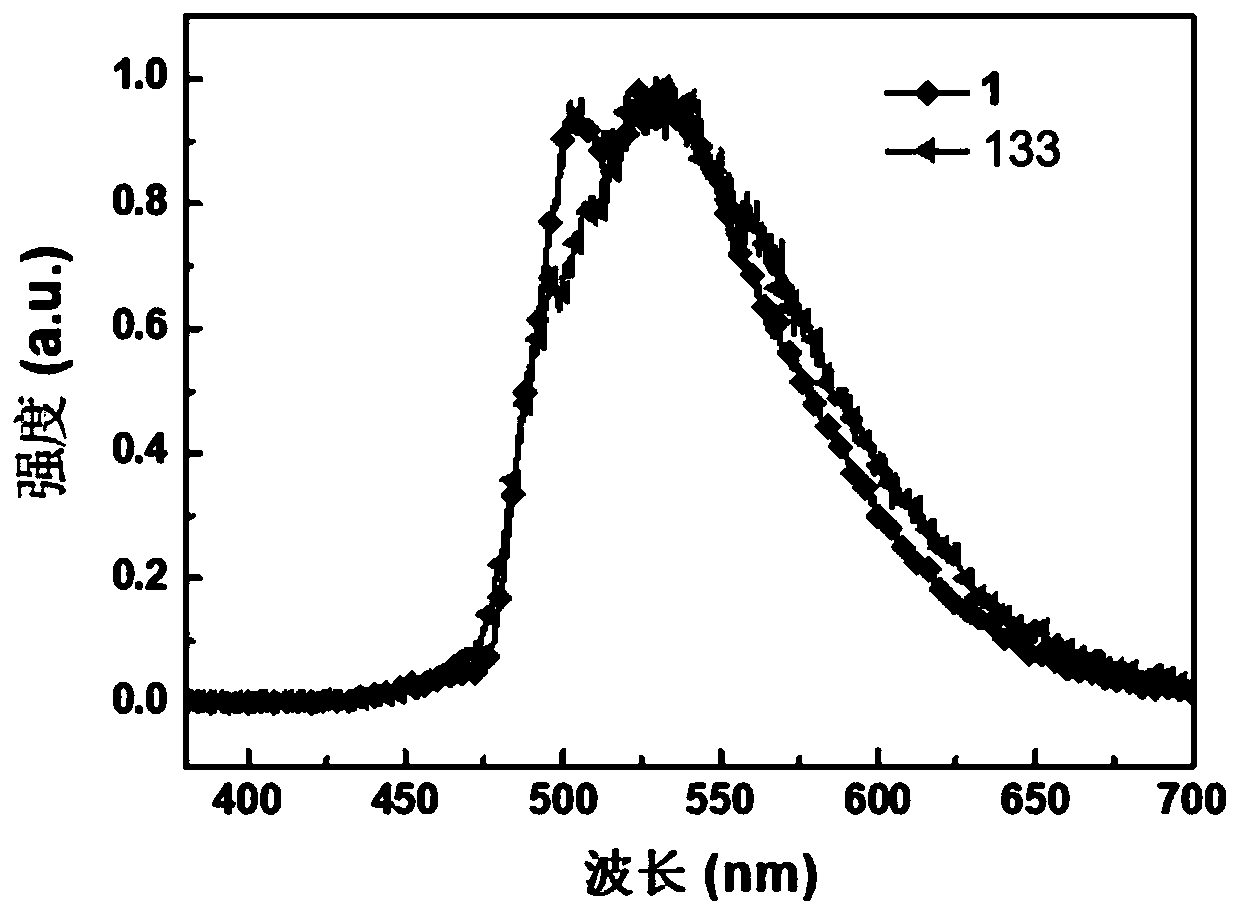
Polycyclic organoboron derivative and electronic device
An electronic device and organoboron technology, applied in the fields of polycyclic organoboron derivatives and electronic devices, achieves the effects of simple preparation method, broad industrialization prospect and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Embodiment 1: the synthesis of compound 1
[0157] (Synthesis of Intermediate 1-1)
[0158]
[0159] Under a nitrogen atmosphere, add 2,6-difluoroiodobenzene (10g, 41.67mmol), dibenzofuran-1-ol (16.9g, 91.67mmol), and potassium carbonate to a three-necked flask equipped with a reflux condenser. (23g, 166.68mmol) and 200mL N-methylpyrrolidone (NMP), and heated to reflux for 6h. After the reaction, the system was cooled to room temperature. A large amount of water was added to form a white precipitate, which was collected by suction filtration. The precipitate was washed successively with water and methanol (50% (V / V)). Finally, the obtained filter cake was dissolved in an appropriate amount of dichloromethane, and further purified by column chromatography (mobile phase: petroleum ether:dichloromethane=3:1 (V / V)) to obtain 20 g of white solid with a yield of 83%. MS (EI): m / z: 568.28 [M + ]. Anal.calcd for C 30 h 17 IO 4 (%): C 63.40, H 3.01; found: C 63.38, H...
Embodiment 2
[0164] Embodiment 2: the synthesis of compound 26
[0165] (Synthesis of Intermediate 1-2)
[0166] The synthetic route of intermediate 1-2 is shown below:
[0167]
[0168] Under nitrogen atmosphere, compound 1 (1.9 g, 4.2 mmol) was dissolved in 200 mL of chloroform and cooled to 0°C. To the above system, 10 mL of a chloroform solution of N-bromosuccinimide (NBS) (822 mg, 4.6 mmol) was added dropwise. After the dropwise addition was completed, the cooling bath was removed, the system rose to room temperature by itself, and the reaction was continued overnight at room temperature. After the reaction was completed, the reaction solution was poured into water, extracted with dichloromethane, and the organic phase was washed with water, 1N sodium hydroxide solution, and water successively. After the organic phase was dried, the solvent was removed under reduced pressure, and the resulting crude product was recrystallized from absolute ethanol to obtain 2.0 g of a light yell...
Embodiment 3
[0173] Embodiment 3: the synthesis of compound 133
[0174] (Synthesis of Intermediate 133-1)
[0175] The synthetic route of intermediate 133-1 is shown below:
[0176]
[0177] Under a nitrogen atmosphere, add 2,6-difluoroiodobenzene (10g, 41.67mmol), dibenzofuran-4-ol (16.9g, 91.67mmol), and potassium carbonate to a three-necked flask equipped with a reflux condenser. (23g, 166.68mmol) and 200mL N-methylpyrrolidone (NMP), and heated to reflux for 6h. After the reaction, the system was cooled to room temperature. A large amount of water was added to form a white precipitate, which was collected by suction filtration. The precipitate was washed successively with water and methanol (50% (V / V)). Finally, the obtained filter cake was dissolved in an appropriate amount of dichloromethane, and further purified by column chromatography (mobile phase: petroleum ether:dichloromethane=3:1 (V / V)) to obtain 22 g of a white solid with a yield of 93%. MS(EI): m / z: 568.32[M + ]. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com