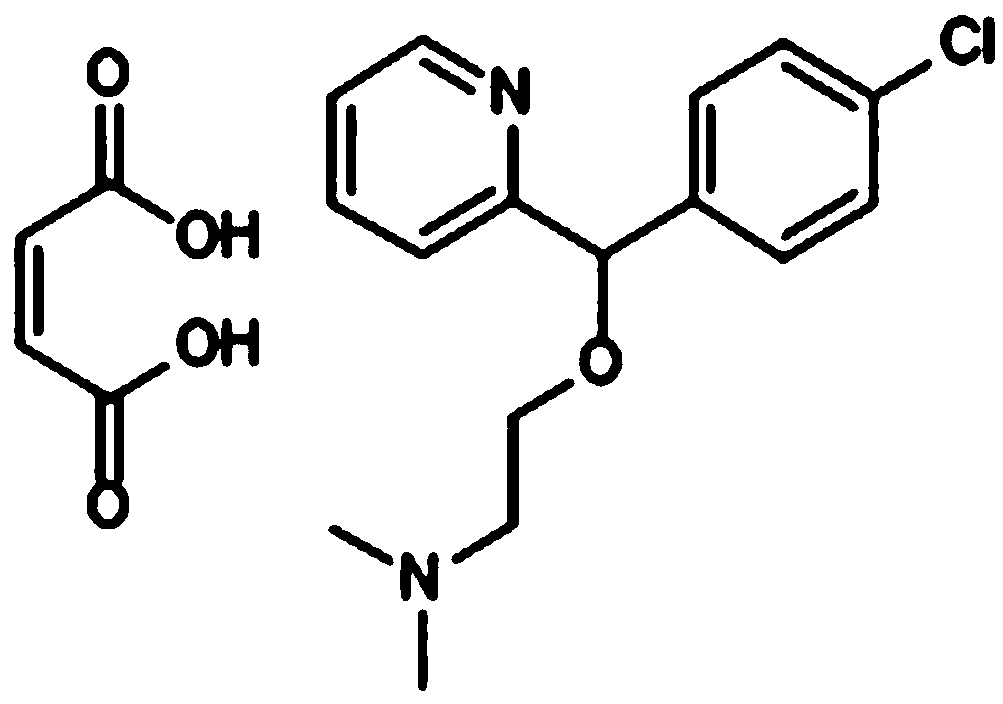
Application of carbinoxamine maleate in preparation of anti-influenza virus medicine
A carbinoxamine maleate, anti-influenza virus technology, applied in the field of medicine, can solve the problems of limited use, not widely used, etc., and achieve the effect of wide application prospects, safety, high efficiency and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
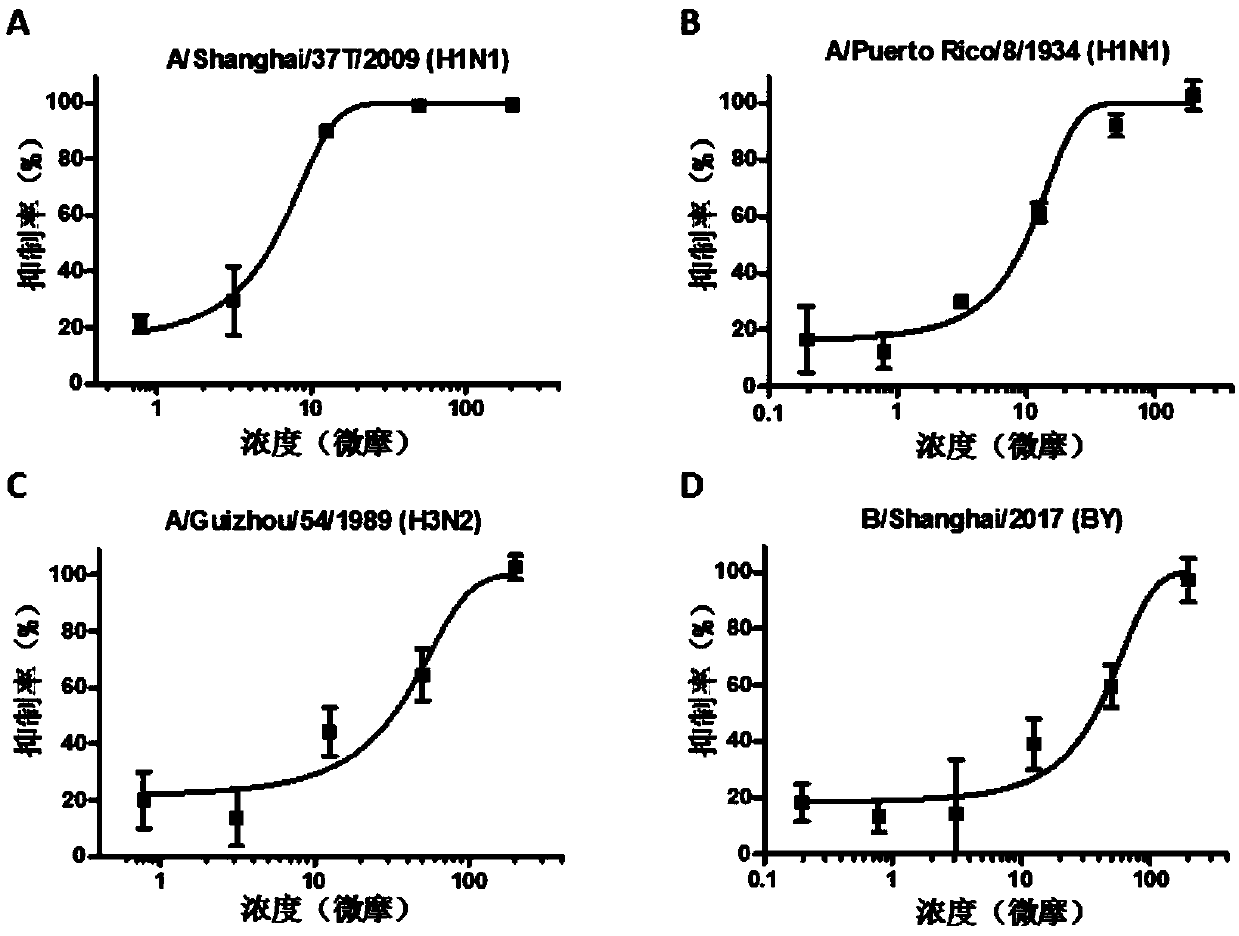
[0031] Example 1 Detection of anti-influenza virus activity of compound CAM in vitro
[0032] The in vitro antiviral experiment of the present invention involves multiple subtypes of influenza A and B viruses, including A / Shanghai / 37T / 2009(H1N1), A / Puerto Rico / 8 / 1934(H1N1), A / Guizhou / 54 / 1989(H3N2) and B / Shanghai / 2017(BY), the specific methods are as follows:
[0033] MDCK cells press 2×10 4 / Well inoculated in 96-well plate at 37℃, 5% CO 2 Culture to a single layer in a constant temperature cell incubator. Add 50μl serially diluted compound and 50μl 100TCID to each well 50 The mixed influenza virus solution infects the cells, and the cells are incubated at 37°C for 8 hours. Discard the mixed solution, add DMEM (containing 2μg / mlTPCK) blank medium 200μl per well, and continue to culture for 48h. Combined with the CCK-8 method, the antiviral activity of the compound is determined by the protective effect of the compound on the cell, and the half effective concentration IC is furth...
Embodiment 2
[0037] Example 2 Detection of Cytotoxicity of Compound CAM
[0038] The CCK-8 method is used to detect the cytotoxicity of compound CAM; the specific method is as follows:
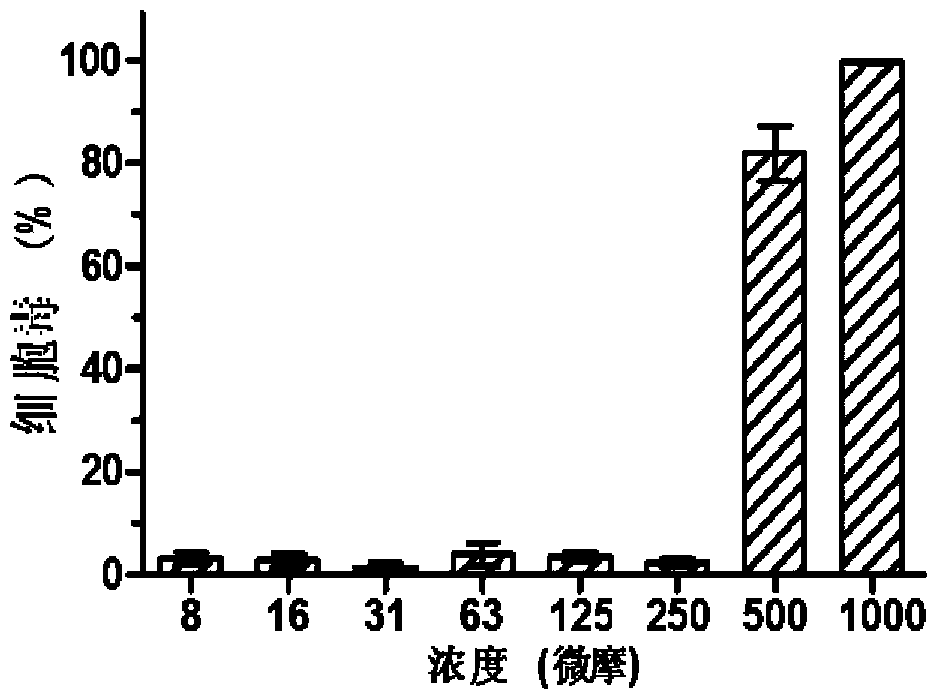
[0039] MDCK cells press 1×10 4 / Well inoculated in 96-well plate at 37℃, 5% CO 2 Cultured to a single layer in a constant temperature cell incubator, add DMEM gradient dilution CAM to a 96-well plate, 100μl per well, continue culturing for 72h, add 20μL of CCK-8 solution to each well, incubate at 37°C for 1h, using multifunctional The microplate reader (Ultra 384, Tecan, NC) detects the absorbance at 450nm, and uses the cell survival rate as an indicator of the toxicity of CAM to MDCK cells; the test results show that the compound CAM has low cytotoxicity and high safety, such as figure 2 As shown, the compound CAM is less toxic to cells and has almost no toxicity to MDCK cells within the concentration range of 250 μM. Its half toxic concentration (CC 50 ) Is 240.52±12.53μM ( image 3 ); The highest drug concen...
Embodiment 3
[0040] Example 3 Detection of anti-influenza virus activity of compound CAM in vivo
[0041] The present invention further evaluates the in vivo inhibitory activity of CAM against influenza virus, using 4 to 8 weeks of C57BL / 6 female mice infected with influenza A virus H7N9 1 hour to intervene in CAM, and detecting the survival rate 14 days after infection, the specific method is as follows:
[0042] A 10LD50 influenza A virus A / Shanghai / 4664T / 2013 (H7N9) was used to inoculate C57BL / 6 female mice aged 4-8 weeks. After 1 hour of infection, CAM was injected intraperitoneally with high dose (10mg / kg / day) and low dose (1mg / kg / day) respectively for continuous intervention for 5 days, and the neuraminidase inhibitor oseltamivir phosphate (OSE ) (1mg / kg / day) was used as the positive control group, and the PBS group was used as the negative control. The survival rate of mice was calculated at 2, 4, 6, 8, 10, 12 and 14 days after influenza virus infection; Mice infected with influenza vir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com