Camel-derived nano antibody capable of being specifically combined with carbonic anhydrase IX and application of camel-derived nano antibody
A nanobody and carbonic anhydrase technology, applied in the field of biomedicine, can solve the problems of strong immunogenicity of monoclonal antibodies, restrictions on the clinical application of ultrasonic molecular imaging, and large molecular weight of monoclonal antibody-particle complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Eukaryotic expression of CA IX extracellular region
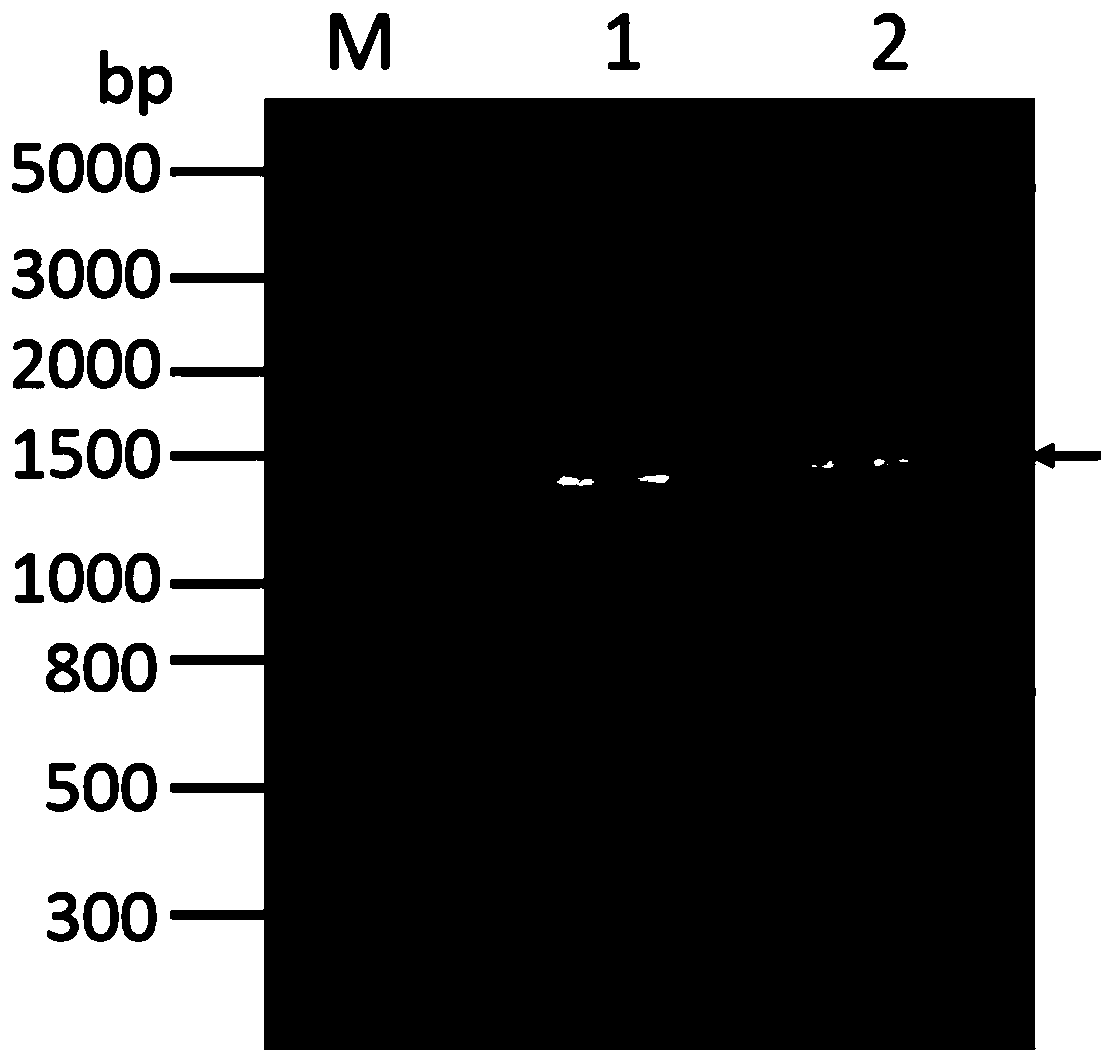
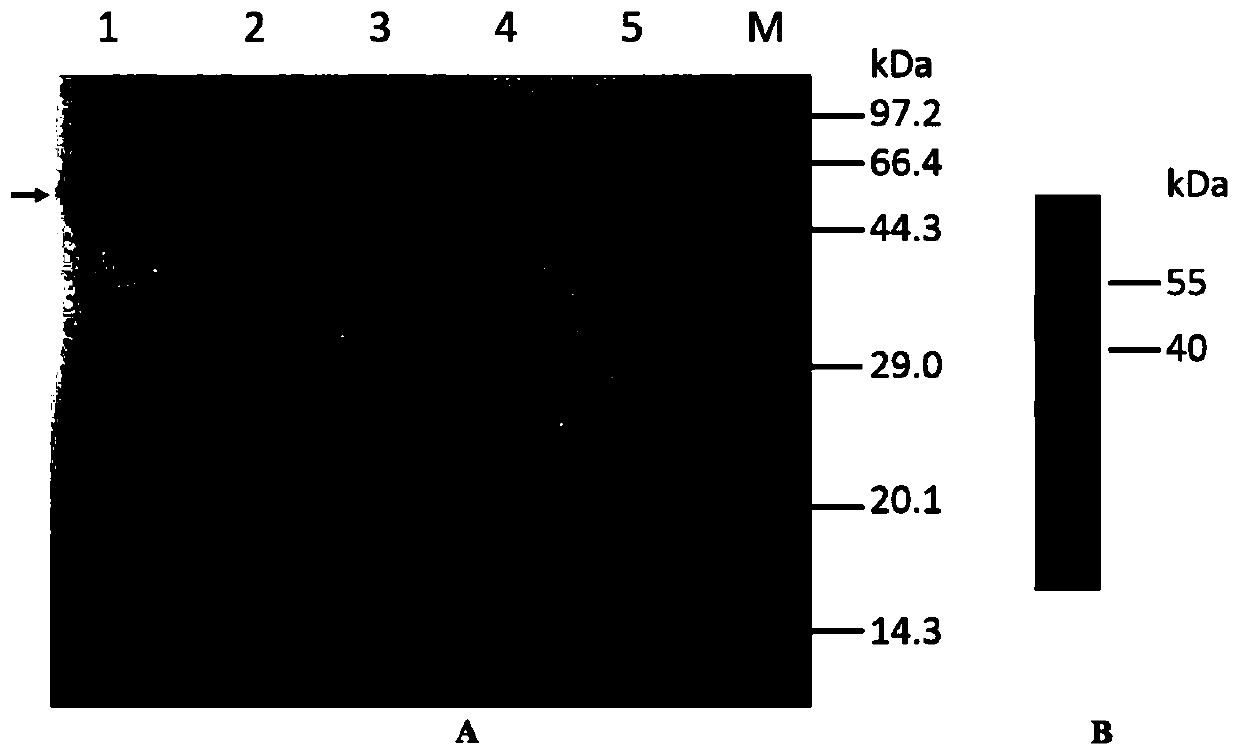
[0069]Using the primer pair CA IXECD-VF (5'-gatcagctagcacagaggttgccccggatgca-3', as shown in SEQ ID No.3) and CA IXECD-VR (5'-catgactcgaggtcaccagcagccaggcag-3', as shown in SEQ ID No.4) from the template plasmid A DNA fragment containing the region from Gln38 to Asp414 of the extracellular region of CA IX (hereinafter referred to as CA IX protein) was amplified from pMDCAIX, and inserted into the eukaryotic expression vector pRAG2a using NheI and XhoI restriction endonucleases. The constructed recombinant plasmid was transformed into competent cells E.coli Top 10, positive clones were identified by colony PCR, and finally the correct plasmid pRAG2a-huCA IXECD was obtained through sequencing verification. After extracting a large amount of the resulting plasmid, use 293fectin to transfect the plasmid DNA into HEK 293F cells for suspension culture and transient expression of the target protein. After the cel...
Embodiment 2
[0071] Example 2 Construction of immune nanobody library
[0072] Two male Xinjiang Bactrian camels about two years old were used for immunization, and the immunization injections were carried out 8 times for a duration of 105 days. The time points of the first two immunizations were day 0 and day 17, respectively, and the amount of antigen per injection per camel was 50 μg and 100 μg, respectively. The subsequent immunization time points were respectively on the 40th, 59th, 74th day and the 87th day, and the amount of antigen injected was 300 μg each time. The last two booster immunizations were completed within 1 week, and the time points were the 102nd and 105th days respectively. One week later, 200 mL of peripheral blood was collected from each camel for immune library construction.
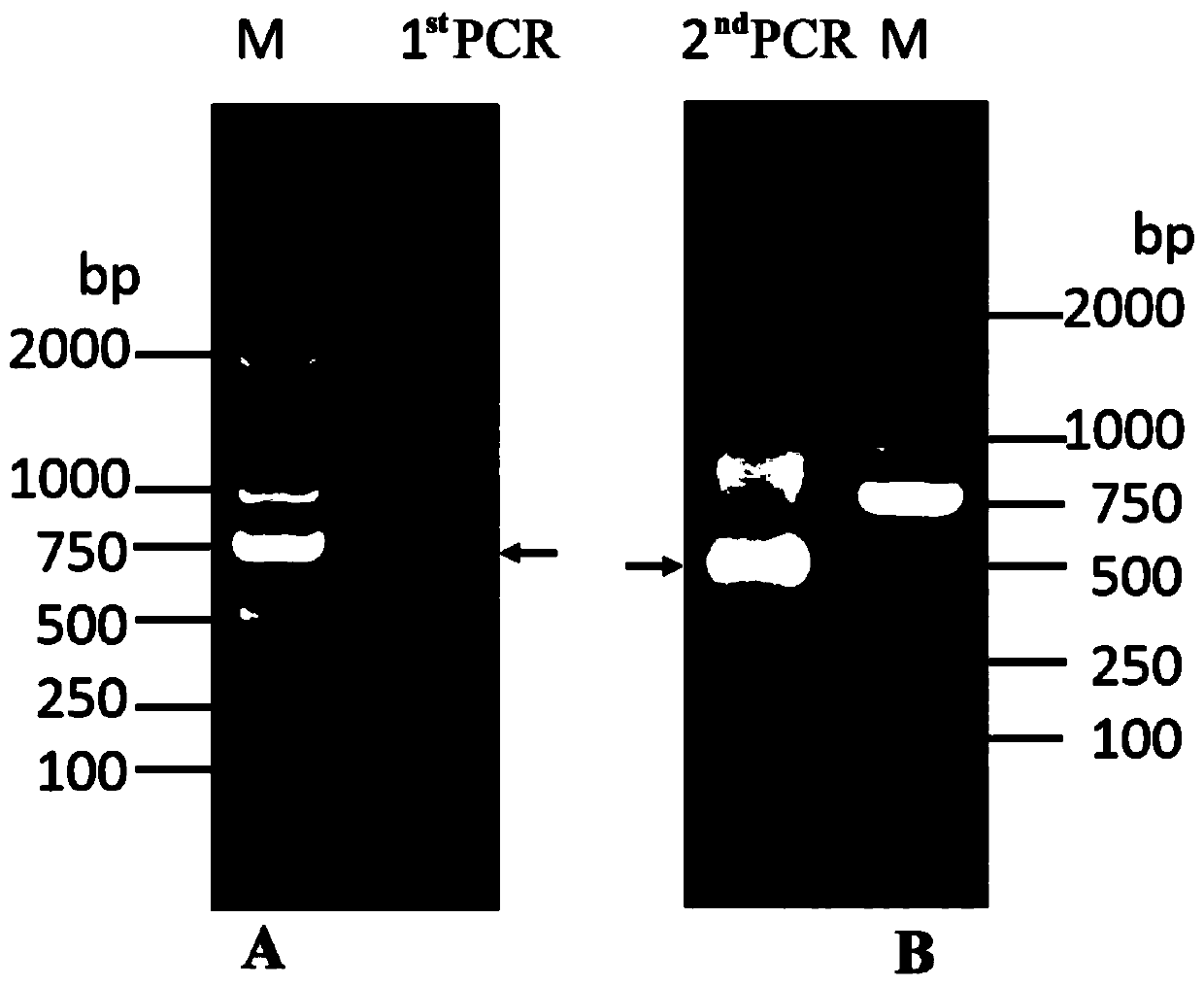
[0073] The total RNA of camel peripheral blood lymphocytes was extracted by T R Izol reagent, the mRNA was extracted, and the mRNA was reverse transcribed into cDNA. Then, the heavy chain...
Embodiment 3
[0075] Example 3 Panning of Nanobodies against CA IX Extracellular Region
[0076] The phage display VHH immune library constructed above was used. Three rounds of in vitro directed screening were performed against the CA IX protein to isolate Nanobodies that specifically bind to CA IX. Briefly, approximately 4 × 10 10 The transformed TG1 cells were inoculated into 2×TY medium containing 100 μg / mL ampicillin and 1% glucose for culture. After 2h, add 4×1010 helper phage M13KO7 and incubate at room temperature for 30min for infection. The infected cells were collected by centrifugation, resuspended in 2×TY medium containing 100 μg / mL ampicillin and 50 μg / mL kanamycin, and cultured overnight at 37°C and 220 r / min. Centrifuge the culture supernatant, add PEG 6 000 / NaCl (20% PEG 6 000 and 2.5mol / L NaCl) solution to precipitate and concentrate the phage particles, and finally resuspend in sterile and pre-cooled PBS buffer.
[0077] The directional screening experiment used the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com