Ketoprofen intermediate, preparation method and applications thereof
A technology of ketoprofen and asana, which is applied in the field of ketoprofen intermediates and its preparation, can solve the problems of highly toxic reaction reagents, harsh reaction conditions, and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Embodiment 1: the preparation of formula II compound
[0179]
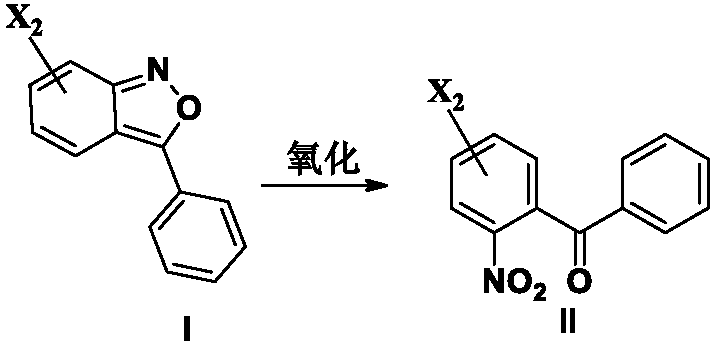
[0180] After adding compound of formula I (13.0g, 0.057mol), DMF (68ml) and water (13.6ml) into the reaction flask, stirring and dissolving, the ozone reaction was started, and the reaction solution gradually became shallower and exothermic, and the temperature rose to 40 ℃ or so. After about 1 hour, a sample was taken for testing. After the raw material disappears, turn off the ozone, change to air or nitrogen for 10-20 minutes, add an appropriate amount of ethyl acetate hydrate, stir, stand still, separate layers, then extract with a small amount of ethyl acetate, and wash the organic layer with water. The organic layer was concentrated to dryness under reduced pressure. 14.6 g of the compound of formula II was obtained, with a content of 93.2% and a yield of 91.9%.
Embodiment 2
[0181] Embodiment 2: the preparation of formula III compound
[0182]
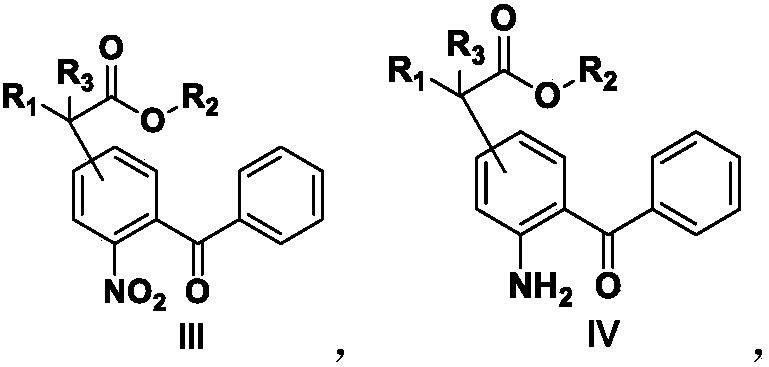
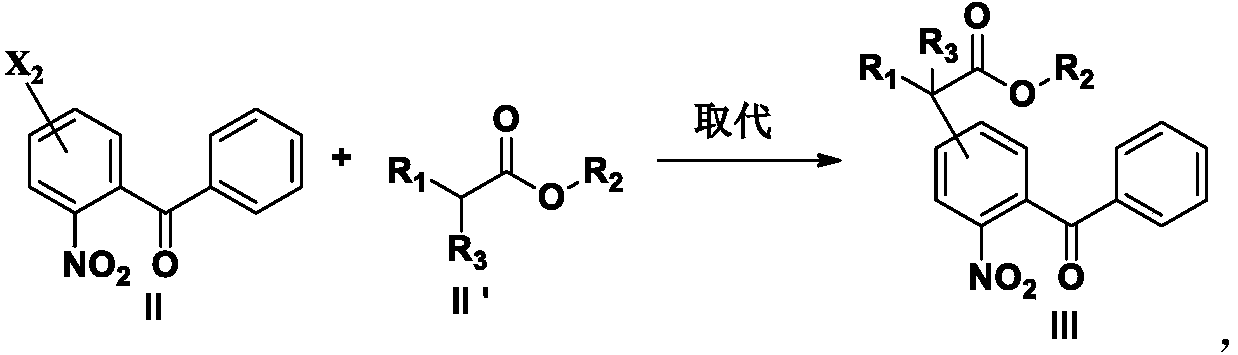
[0183] Add formula II compound (10g, 0.038mol), DMF (50ml), K 2 CO 3 (11g, 0.080mol), start to heat up after stirring evenly, when the temperature is 80°C, add a DMF solution of methyl cyanoacetate (6.6g, 0.066mol) dropwise, and after dripping at 80°C for 30 minutes, take a sample to detect the central control, when After the raw materials disappeared, the temperature was lowered to about 10°C, and the pH was adjusted to 1-2 with 1N dilute HCl, extracted with ethyl acetate, the organic layer was washed with an appropriate amount of water, and concentrated to dryness under reduced pressure to obtain 12.1 g of the compound of formula III, with a content of 87.3%. , yield 85%.
Embodiment 3
[0184] Embodiment 3: the preparation of formula IV compound
[0185]
[0186] Add the compound of formula III (10 g, 0.03 mol), Pd / C (50 mg) and methanol (30 ml) into a 100 ml reaction flask, and stir the reaction with hydrogen gas. After reacting at 25°C for 5 hours, sampling and testing were started. When the raw materials disappeared, suction filtration, washing with an appropriate amount of methanol, and concentration of the mother liquor to dryness under reduced pressure gave 8.97 g of the compound of formula IV, with a content of 97.2% and a yield of 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com