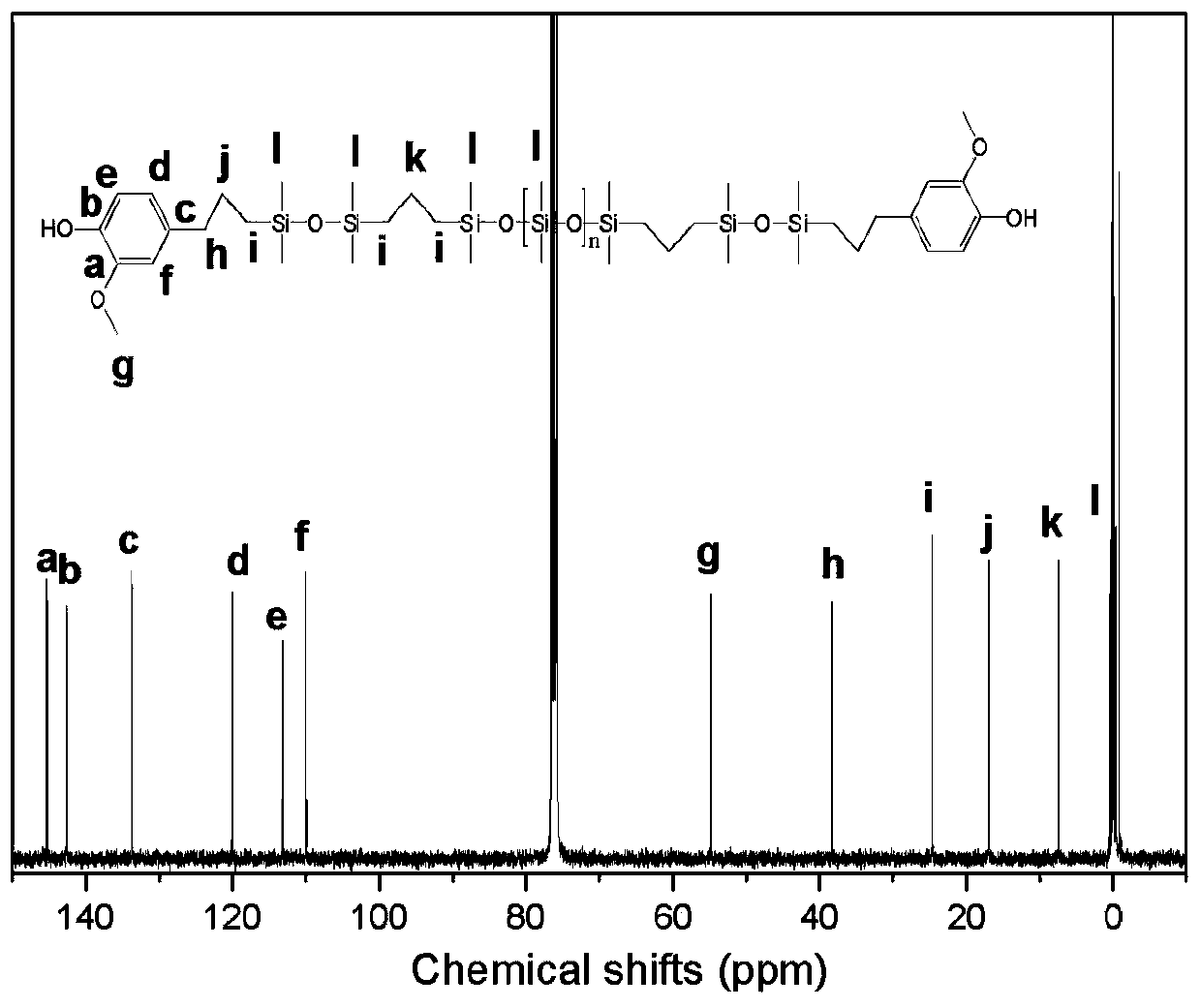
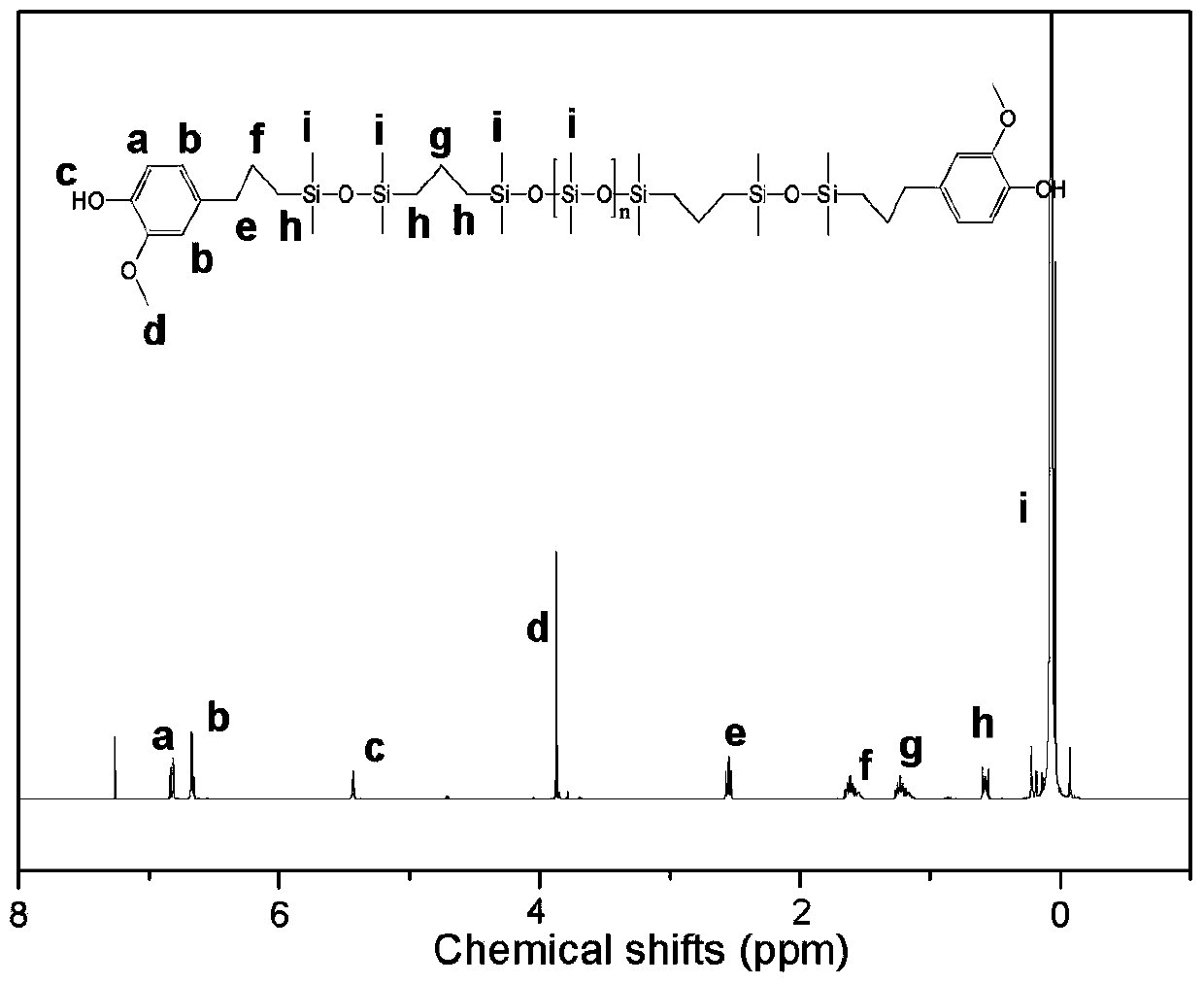
Phenol-terminated polysiloxane, preparation method thereof, and polysiloxane-polycarbonate copolymer
A technology of phenol-terminated polysiloxane and hydrogen silane is applied in the field of preparation of polysiloxane, which can solve the problems of easy yellowing of products, increase process complexity, and no mention of platinum content, and achieve free phenol content. Reduced, good blending and dispersibility, low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 Eugenol modified hydrosilane synthesis
[0055] In a 1L three-necked flask, add 163 parts of eugenol (parts by mass, the same below) and Castel (tetramethyldivinyldisiloxane-platinum complex) catalyst (calculated as eugenol and tetramethyl 1 ppm of the total amount of disiloxane), the temperature was raised to 150° C., 134 parts of tetramethyldisiloxane was slowly added dropwise, and the reaction was continued for 1 h after the dropwise addition was completed. After the reaction is completed, use vacuum distillation, 30 theoretical plates, and a reflux ratio of 1:1 to extract the fraction at 163-169 ° C under a pressure of 50 Pa to obtain eugenol-modified hydrosilane (IIa) with a purity of 99.2%.
Embodiment 2
[0056] Example 2 Synthesis of 4-allylphenol modified hydrosilane
[0057] In a 1L three-necked flask, add 134 parts of 4-allylphenol (parts by mass, the same below) and chloroplatinic acid catalyst (calculated as 20ppm of the total amount of 4-allylphenol and tetramethyldisiloxane by platinum) , at 20°C, 670 parts of tetramethyldisiloxane was slowly added dropwise, and the reaction was continued for 10 h after the dropwise addition was completed. After the reaction is completed, use vacuum distillation, 20 theoretical plates, and a reflux ratio of 1:5 to extract the fraction at 156-162°C under a pressure of 60Pa to obtain 4-allylphenol-modified hydrosilane (IIb), with a purity of 99.5%.
Embodiment 3
[0058] Example 3 Synthesis of eugenol-modified hydrosilane
[0059] In a 1L three-necked flask, add 164 parts of eugenol (parts by mass, the same below) and Castel (tetramethyldivinyldisiloxane-platinum complex) catalyst (calculated as eugenol and tetramethyl 5ppm of the total amount of disiloxane), the temperature was raised to 70°C, and 402 parts of tetramethyldisiloxane was slowly added dropwise, and the reaction was continued for 4 hours after the dropwise addition was completed. After the reaction was completed, use vacuum distillation, 25 theoretical plates, reflux ratio 1:3, extract the fraction at 185-191°C under the pressure of 100Pa to obtain eugenol-modified hydrosilane (IIc) with a purity of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com