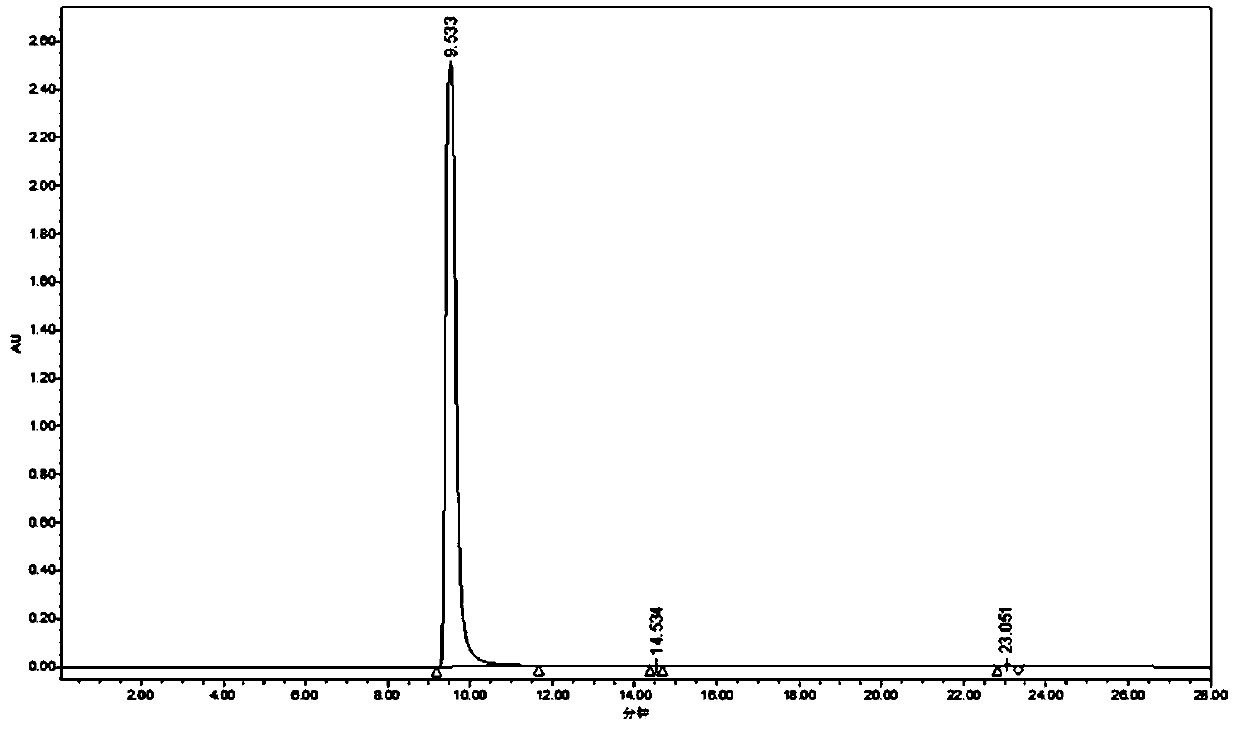
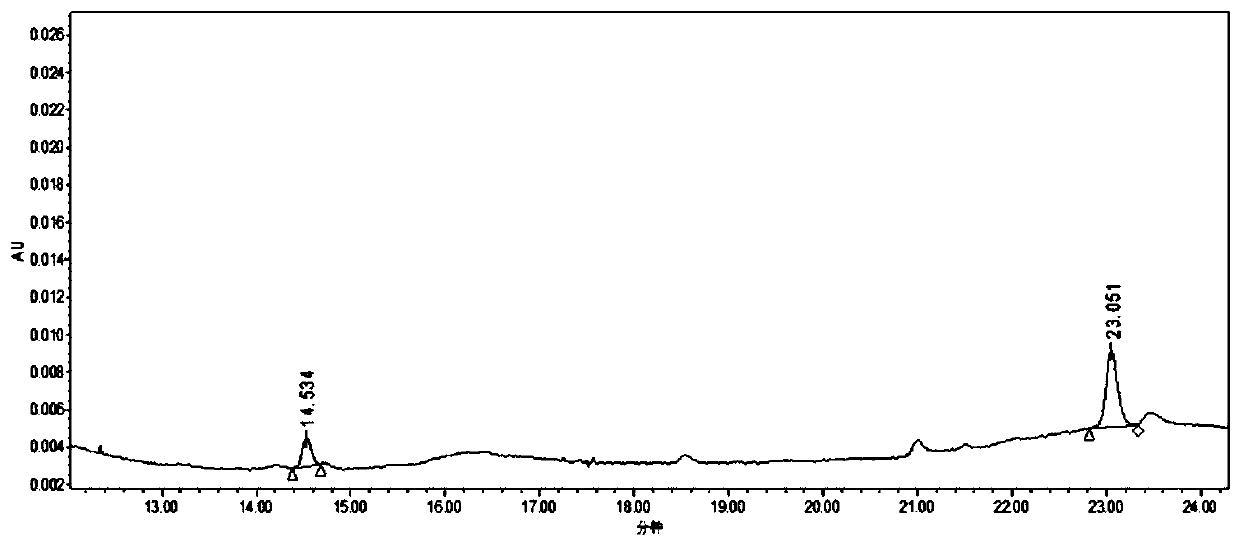
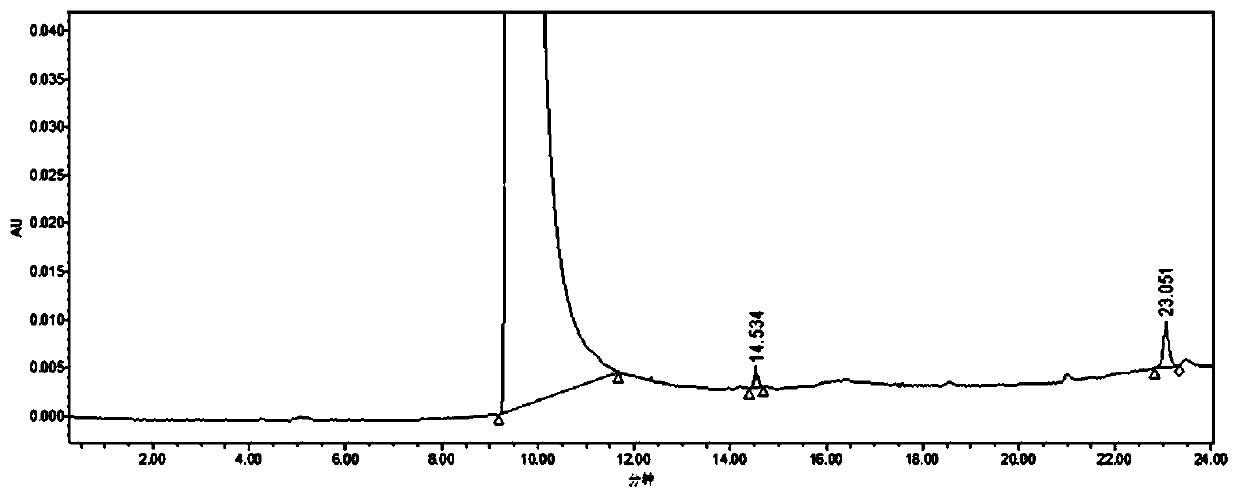
Purification method of 2-methyl-4-isothiazolin-3-ketone
A technology of isothiazoline and purification method, which is applied in organic chemistry and other fields, and can solve problems such as low purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The purification method of the present embodiment 2-methyl-4-isothiazolin-3-one comprises the following steps:
[0025] (1) Mix 25g of 2-methyl-4-isothiazolin-3-one reagent (purity is 95%, purchased from Aladdin Company) with 10mL acetone (chromatographically pure 99.8%, purchased from Merck Company), and heat up to 40°C, stirred until completely dissolved, kept warm for 10 minutes, and filtered to obtain the mixed solution.
[0026] (2) Place the mixed solution in an environment of 25° C. to cool naturally for 3 hours, and then place it in an environment of 4° C. to cool for 5 hours, and then perform suction filtration to obtain crystals.
[0027] (3) The crystals were washed 3 times with petroleum ether.
[0028] The above steps (1), (2) and (3) are all completed in an inert gas glove box, and the inert gas is nitrogen.
[0029] (4) The washed crystals were subjected to vacuum freeze-drying at a temperature of -80° C., a vacuum degree of <0.12 Mbar, and a drying tim...
Embodiment 2
[0031] The purification method of the present embodiment 2-methyl-4-isothiazolin-3-one comprises the following steps:
[0032] (1) 25g of 2-methyl-4-isothiazolin-3-one reagent (purity is 95%, Bailingwei Technology Co., Ltd.) is mixed with 25mL ethyl acetate (chromatographically pure 99.9%, purchased from Merck Co.), and the temperature is raised to 50°C, stir until completely dissolved, keep warm for 20 minutes, and filter to obtain the mixture.
[0033] (2) Place the mixed solution in an environment of 20° C. to cool naturally for 3 hours, and then place it in an environment of 8° C. to cool for 5 hours, and then perform suction filtration to obtain crystals.
[0034] (3) The crystals were washed 3 times with petroleum ether.
[0035] The above steps (1), (2) and (3) are all completed in an inert gas glove box, and the inert gas is argon.
[0036] (4) The washed crystals were subjected to vacuum freeze-drying at a temperature of -40° C., a vacuum degree of <0.12 Mbar, and a...
Embodiment 3
[0038] The purification method of the present embodiment 2-methyl-4-isothiazolin-3-one comprises the following steps:
[0039] (1) 25g of 2-methyl-4-isothiazolin-3-one reagent (purity is 95%, Bailingwei Technology Co., Ltd.) is mixed with 25mL ethyl acetate (chromatographically pure 99.9%, purchased from Merck Co.), and the temperature is raised to 45°C, stirred until completely dissolved, kept warm for 15 minutes, and filtered to obtain the mixture.
[0040] (2) The mixture was naturally cooled at 23°C for 3 hours, then cooled at 0°C for 5 hours, and suction filtered to obtain crystals.
[0041] (3) The crystals were washed 3 times with petroleum ether.
[0042] The above steps (1), (2) and (3) are all completed in an inert gas glove box, and the inert gas is nitrogen.
[0043] (4) The washed crystals were subjected to vacuum freeze-drying at a temperature of -80° C., a vacuum degree of <0.12 Mbar, and a drying time of 4 days, and finally 21.2 g of snow-white crystals were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com