CFAN catalyst, preparation method thereof, and application of CFAN catalyst in production of hydrogen from methane
A catalyst, methane technology, used in heterogeneous catalyst chemical elements, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., to improve stability and catalytic activity, inhibit sintering and agglomeration, The effect of suppressing sintering and agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
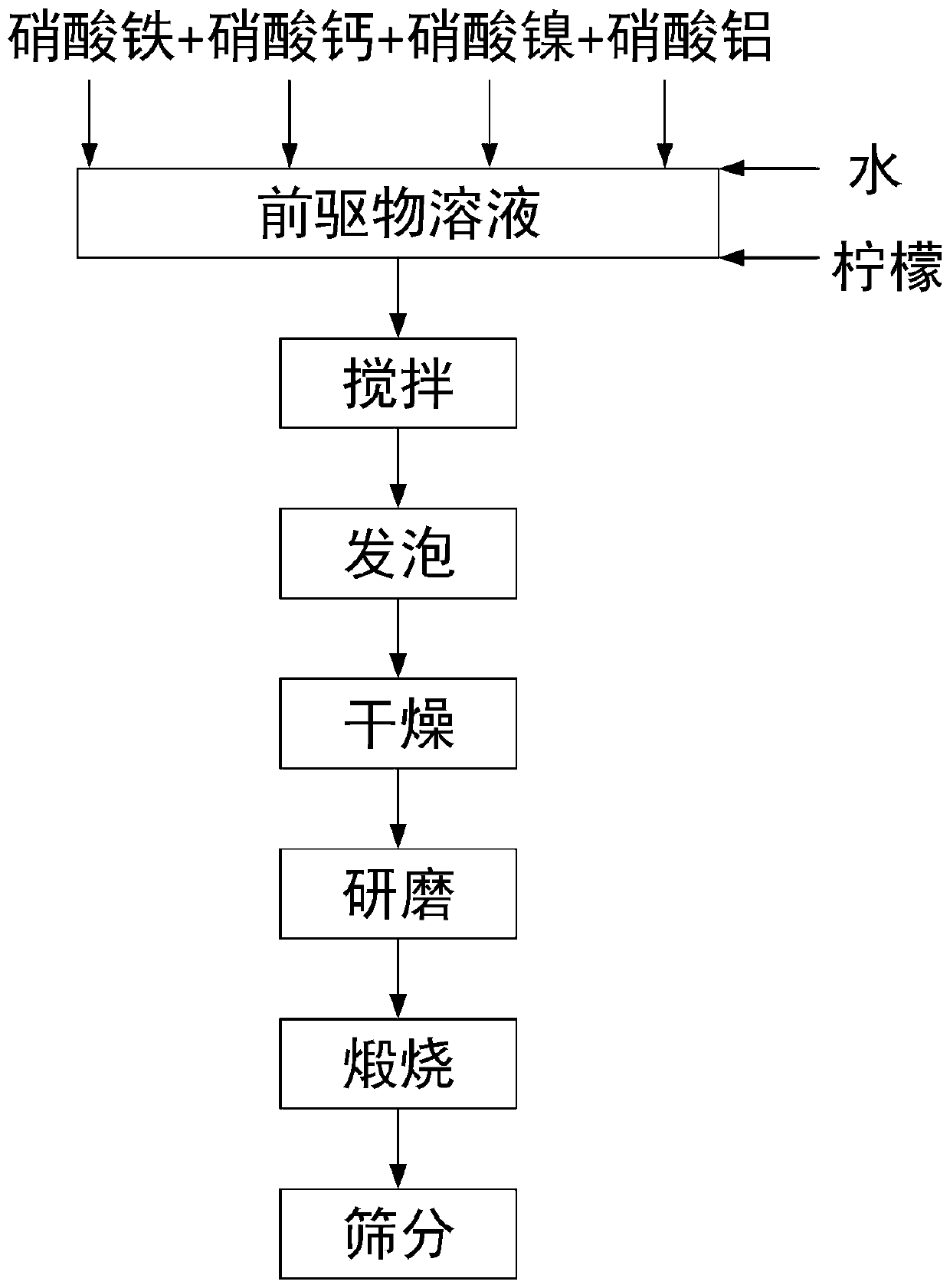
[0065] 1) Mix iron nitrate, calcium nitrate, nickel nitrate, aluminum nitrate and citric acid (in the material, the molar ratio of Ca-Fe-Al-Ni element is 4:4:1:1), and the molar addition amount of citric acid is all 1.3 times the total molar weight of metal atoms;
[0066] 2) Add deionized water to configure a solution, satisfying that the concentration of nickel nitrate solution in the mixed solution is 0.10mol / L;
[0067] 3) Stir the prepared solution at 40°C for 30 minutes;
[0068] 4) Put the solution obtained in step 3) in a drying oven, foam and dry for 5 hours at a temperature range of 180° C., and crush and grind the obtained solid sample;
[0069] 5) Put the ground sample in step 4) into a muffle furnace, and calcinate it at 850° C. for 4 hours in an air atmosphere. The heating rate is guaranteed to be 2.5° C. / min, and the calcined solid powder is ground to The particle size is less than 0.3 mm, and the CFAN catalyst is finally obtained, and the catalyst is ground t...
Embodiment 2
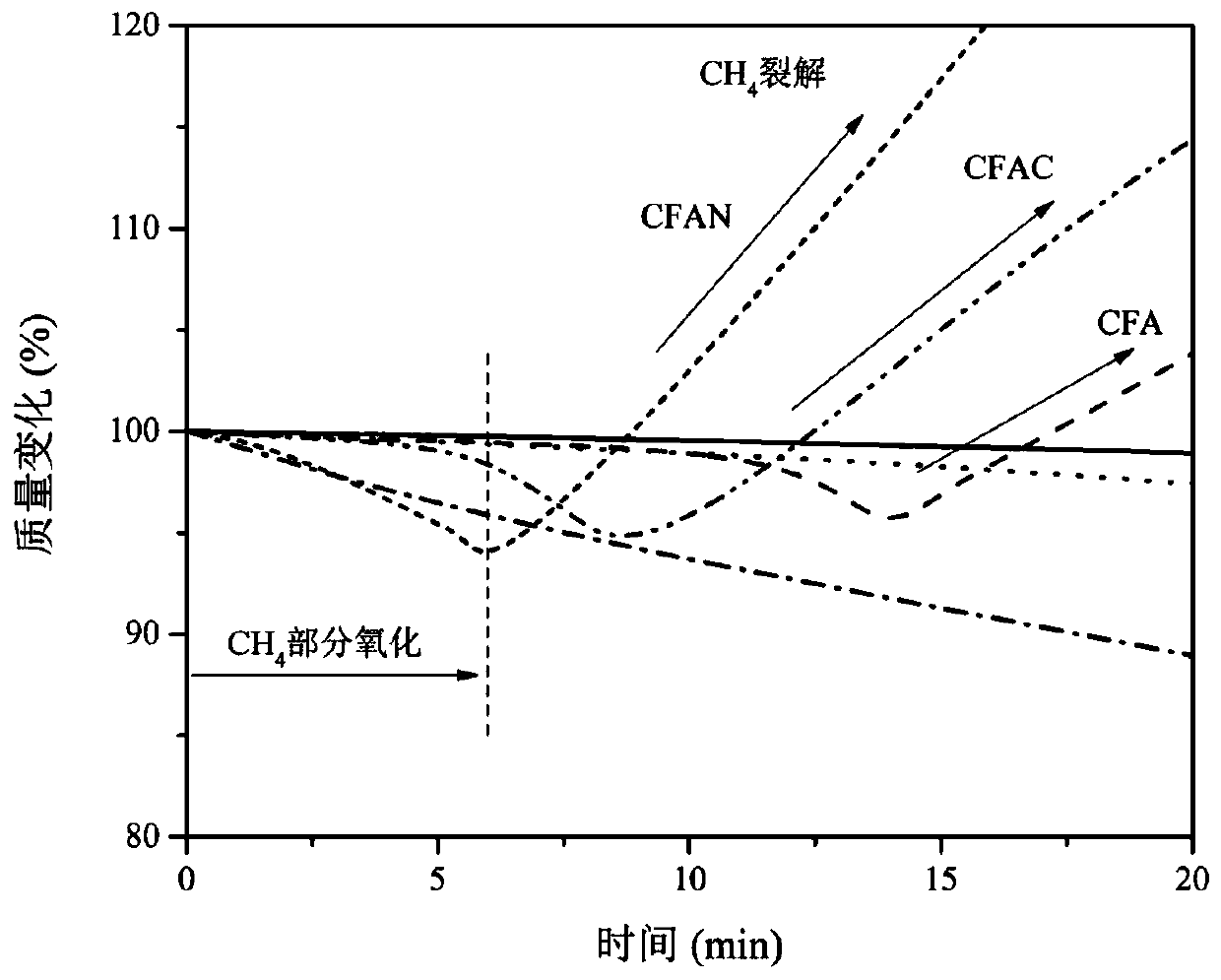
[0123] Compared with Example 1, the only difference is that the methane catalytic reaction temperature is 600-850°C. Determination of methane hydrogen production rate, product hydrogen purity and methane conversion rate, the results are shown in Figure 8 ~ Figure 10 .
[0124] After determining the best catalyst, we optimized the reaction temperature range, using hydrogen production rate, hydrogen concentration and methane conversion as indicators, the results are as follows Figure 8 , Figure 10 shown. From the graph of hydrogen yield and hydrogen concentration results in the temperature range of 600-850°C, it can be found that when the temperature is lower than 700°C, both the hydrogen yield and the hydrogen concentration will drop significantly in performance. When the temperature is higher than 800°C, it can be found that the catalyst has good performance at the initial stage of the reaction, but too high a temperature will cause rapid agglomeration and sintering of t...
Embodiment 3
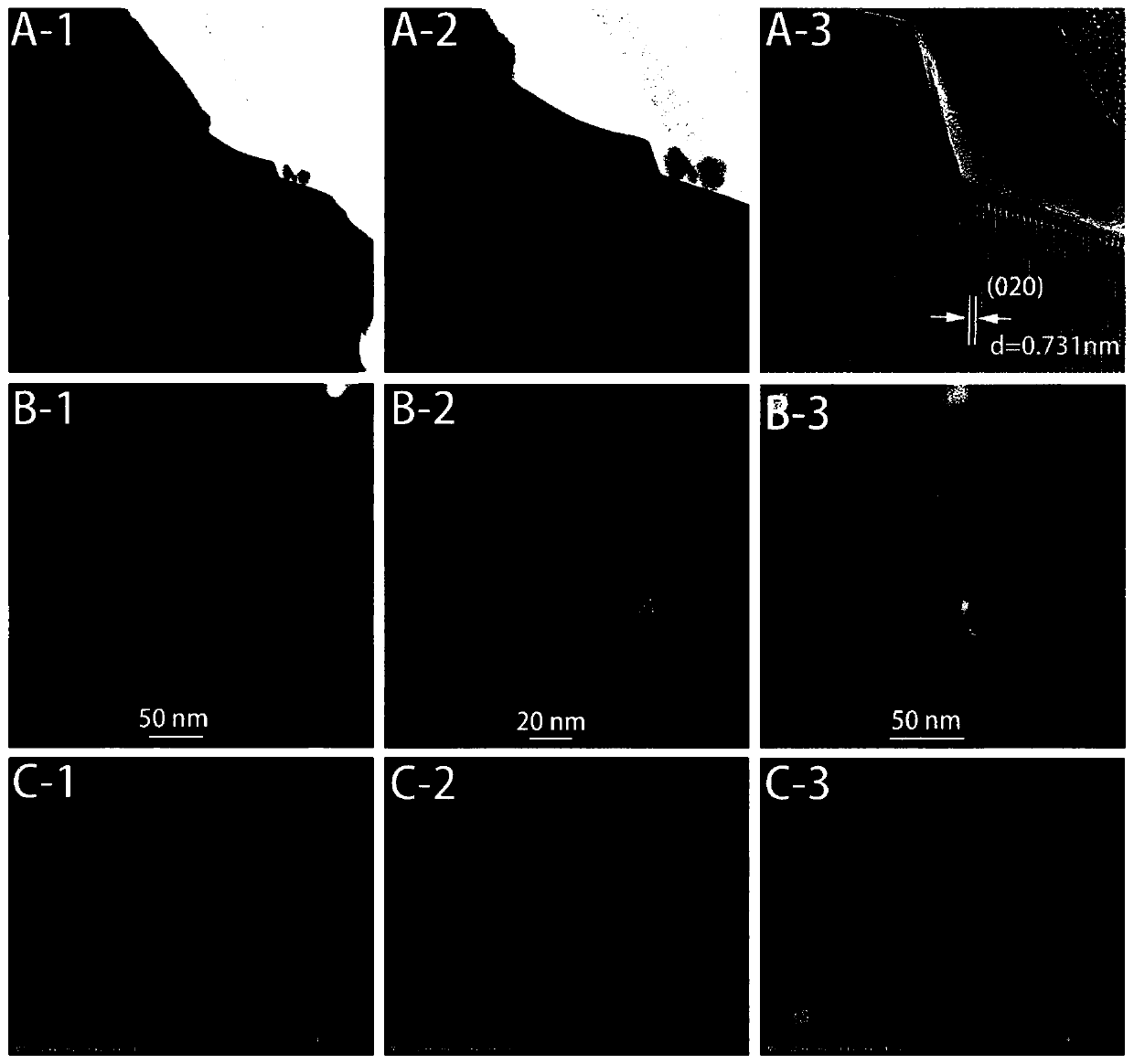
[0126] Compared with Example 1, the newly prepared CFAN→half-hour catalytic methane cracking CFAN→one-hour catalytic methane cracking CFAN, a slight reduction of the carrier occurs, and the coefficient y is from 1.52→1.40→1.28, which proves the trace reduction of the carrier. The coefficient y gradually decreases from large to small, but the methane cracking activity of the process does not change significantly, such as Figure 11 , Figure 12 As shown, the oxygen carrier still maintains high catalytic activity for methane cracking, that is, high hydrogen production rate and concentration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com