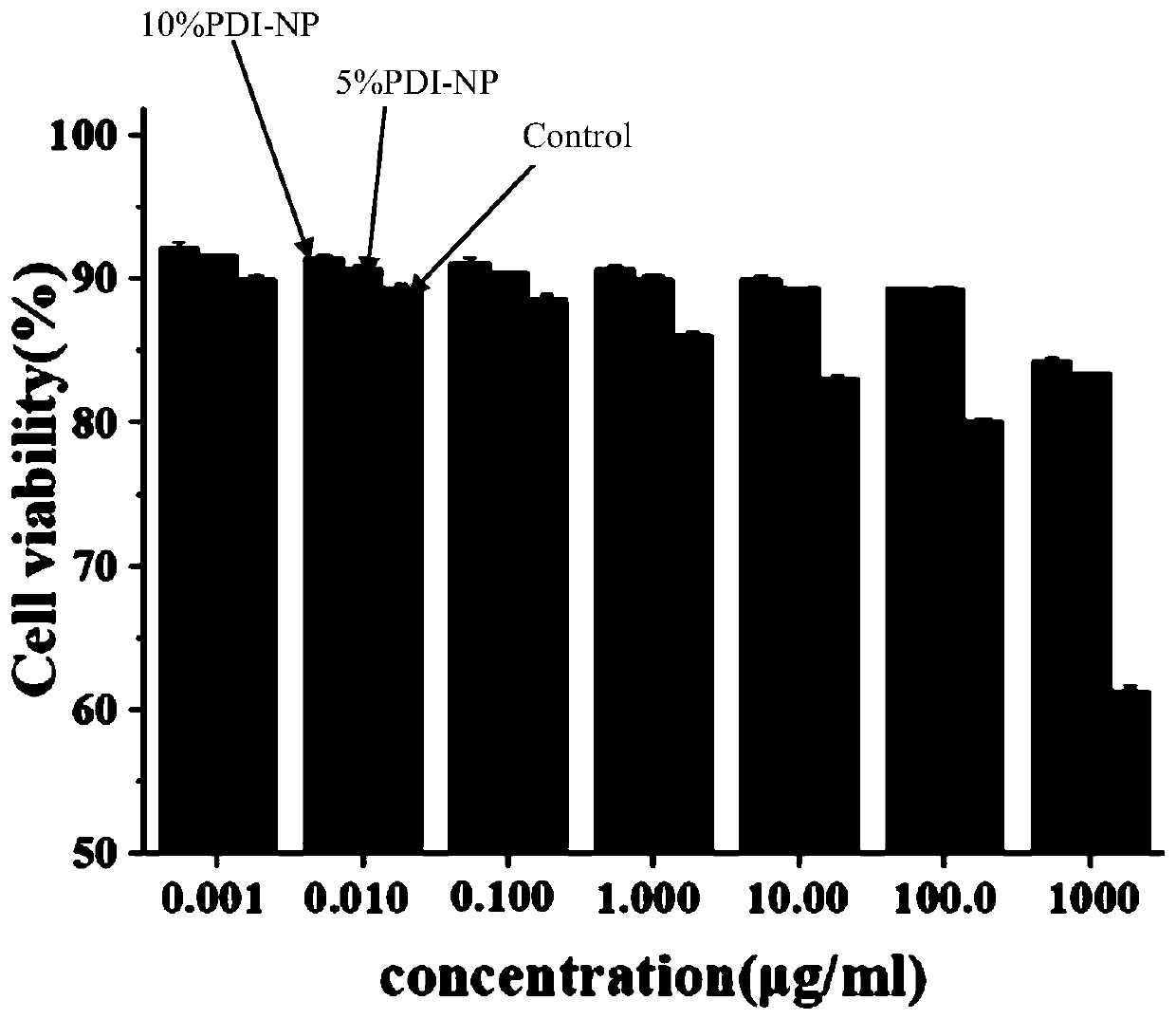
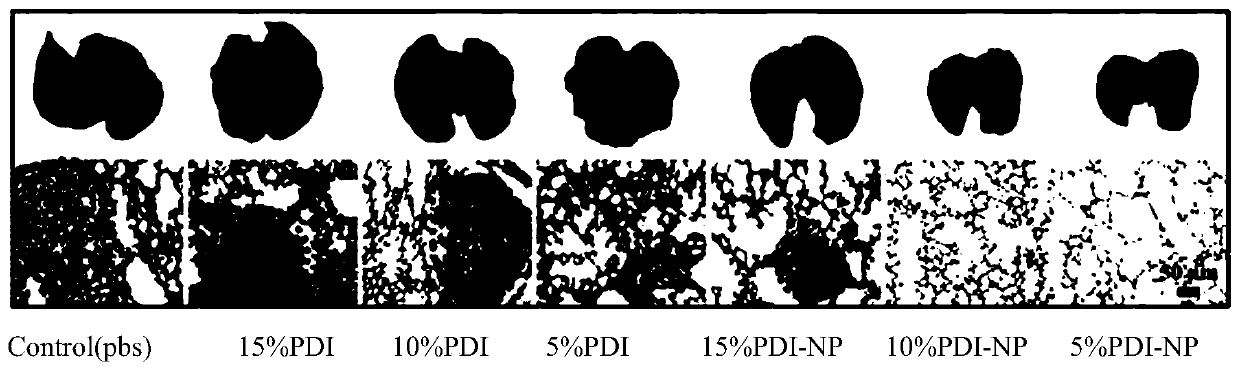
Method for preparing lyserin-based near-infrared material
A near-infrared, lyoline-based technology, used in medical preparations containing active ingredients, wave energy or particle radiation treatment materials, pharmaceutical formulations, etc., can solve the problems of weak gene carrying capacity, poor biocompatibility, etc. Secondary damage, strong antibacterial ability, beneficial to deep penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for preparing a phenoline-based near-infrared material, comprising the following steps: (1), synthesizing a phenoline-based monochloro-substituted perylene diacid anhydride compound: a, dissolving the perylene diacid anhydride in concentrated sulfuric acid, with a mass ratio of 1: 2.3 Until it is completely dissolved, mix evenly, complete under the condition of magnetic stirring in a nitrogen atmosphere, the volume concentration of concentrated sulfuric acid is 67%, b, the mixed solution obtained in step a is at -0.5°C, slowly add TBCA of concentrated sulfuric acid Solution, keep the reaction for 2.7h, and then detect the degree of reaction; c, when the alcohol phase appears, the chromatographic column separates the large molecular weight product, and the deionized water is purified by dialysis for 28h. , after freeze-drying, green powder is obtained, that is, lethrin-based monochloro-substituted perylene diacid anhydride compound; (2), preparation of lethrin-ba...
Embodiment 2
[0043] The difference from the above-mentioned Example 1 is that a preparation method of a phenoline-based near-infrared material comprises the following steps: (1), synthesizing a morpholine-based monochloro-substituted perylene diacid anhydride compound: a, dissolving the perylene diacid anhydride in In concentrated sulfuric acid, the mass ratio is 0.7:2.2 until it is completely dissolved, and mixed evenly. In a nitrogen atmosphere, it is completed under magnetic stirring conditions. The volume concentration of concentrated sulfuric acid is 65%. b. The mixed solution obtained in step a is kept at -1°C Next, slowly add the TBCA solution of concentrated sulfuric acid dropwise, keep the reaction for 2.5h, and then detect the degree of reaction; c, when the alcohol phase appears, the chromatographic column separates the large molecular weight product, and the deionized water is dialysis purified for 24h, and the dialysis purification part is dialysis membrane Molecular weight cut...
Embodiment 3
[0046]The difference from the above-mentioned Example 1 is that a preparation method of a phenoline-based near-infrared material comprises the following steps: (1), synthesizing a morpholine-based monochloro-substituted perylene diacid anhydride compound: a, dissolving the perylene diacid anhydride in In concentrated sulfuric acid, the mass ratio is 1.2:2.4 until it is completely dissolved, and the mixture is evenly mixed. In a nitrogen atmosphere, it is completed under the condition of magnetic stirring. The volume concentration of concentrated sulfuric acid is 70%. b. Mix the solution obtained in step a at 0°C , slowly drip the TBCA solution of concentrated sulfuric acid, keep the reaction for 3h, and then detect the degree of reaction; c, when the alcohol phase appears, the chromatographic column separates the large molecular weight product, and the deionized water is purified by dialysis for 30h, and the molecular weight cut-off of the dialysis membrane at the dialysis purif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com