Application of isoindolone compound in preparation of medicine for preventing and treating osteolytic diseases
A subunit vaccine and genetic engineering technology are applied in the field of genetic engineering subunit vaccines for preventing new variant strains of infectious bursal virus of chickens and the preparation thereof, and can solve the problems of inability to control infection well, atrophy of bursal organs, There are no clinical symptoms and other problems, and the effect of preventing the immune effect is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Construction of IBDV FJ-1812 strain VP2 protein gene cloning vector
[0033] 1. Extraction of RNA from disease materials
[0034] Main reagents: ultrapure total RNA extraction kit (Simgen product).
[0035] Operation process: Take about 100mg of chicken bursa tissue infected with the mutant strain of infectious bursal virus, and add 1ml of Trizol to a sterile 1.5mL Ep tube. Add 2 3mm stainless steel grinding balls. Place in a tissue grinder pre-cooled at -30°C and run at a vibration frequency of 50 Hz for 60 s. Add 200 μL of chloroform, shake vigorously to mix, and centrifuge at 12000 g for 15 min at 4°C. Take about 600 μL of the supernatant, add an equal volume of 70% ethanol, and mix well. Transfer 600 μL of the above mixture to a nucleic acid purification column, centrifuge at 12,000 g for 1 min at 4°C, and discard the filtrate. Then add the remaining mixture to the nucleic acid purification column, centrifuge at 12000g for 1min at 4°C, and discard the...
Embodiment 2
[0063] Example 2 IBDV FJ-1812 strain VP2 prokaryotic expression
[0064] 1. Construction and expression of E.coli BL21 / pET28a IBDV FJ-1812 VP2
[0065] The pTOPO-IBDV FJ-1812 VP2 plasmid was digested with a restriction endonuclease (BamHI / Xho I), and ligated with the expression plasmid pET28a digested with the same restriction endonuclease. The ligation product was named: pET28aIBDV FJ-1812 VP2.
[0066] The ligation product was used to transform Escherichia coli BL21(DE3), and transformants were screened. An engineering bacterium expressing IBDV FJ-1812 VP2 antigen protein was constructed. Name: E.coli BL21 / pET28a IBDV FJ-1812 VP2.
[0067] Induced expression experiments were carried out, the inducer was α-lactose, and the working concentration was: 0.03mol / L. Induced products were detected by SDS-PAGE electrophoresis. The results showed that E.coli BL21 / pET28a IBDV FJ-1812 VP2 had no obvious protein expression band.
[0068] 2. Optimization of expression codons of IBDV...
Embodiment 3
[0088] Example 3 Detection and Analysis of Escherichia coli BL21(DE3) / pET28a IBDV FJ-1812 VP2M3 Expression Protein
[0089] 1. Induced expression and molecular weight determination
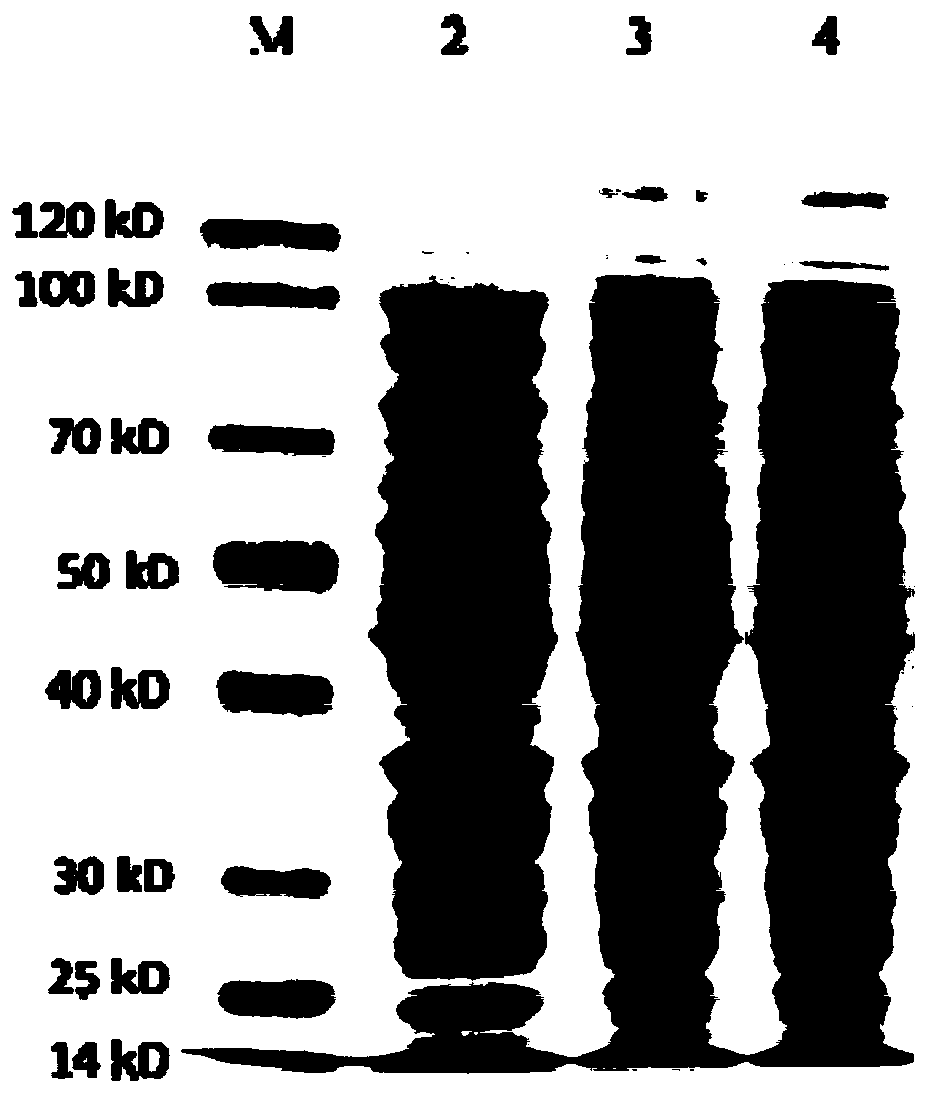
[0090] The protein expression engineered bacteria constructed in this example were induced to express. The inducer is α-lactose, and the working concentration is: 0.03mol / L. Then, after ultrasonic centrifugation, the protein expression and dissolution properties were detected by SDS-PAGE electrophoresis. The results showed that the expression and dissolution properties of the target protein were better. The expression amount is about 7%-10% of the total protein, and the main expressed protein is stored in the supernatant after being centrifuged at 12000RPM for 10min, indicating that it is a soluble protein (see figure 1 ). Because the molecular weight of the expressed protein is very close to that of a self-protein of Escherichia coli, the molecular weight of the expressed protein is confirmed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com