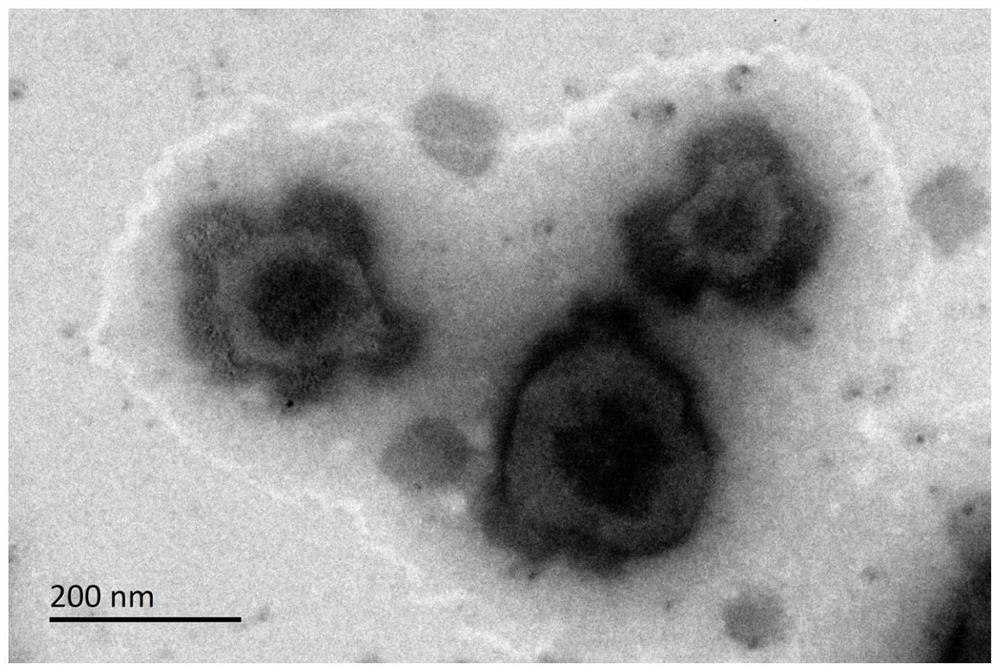
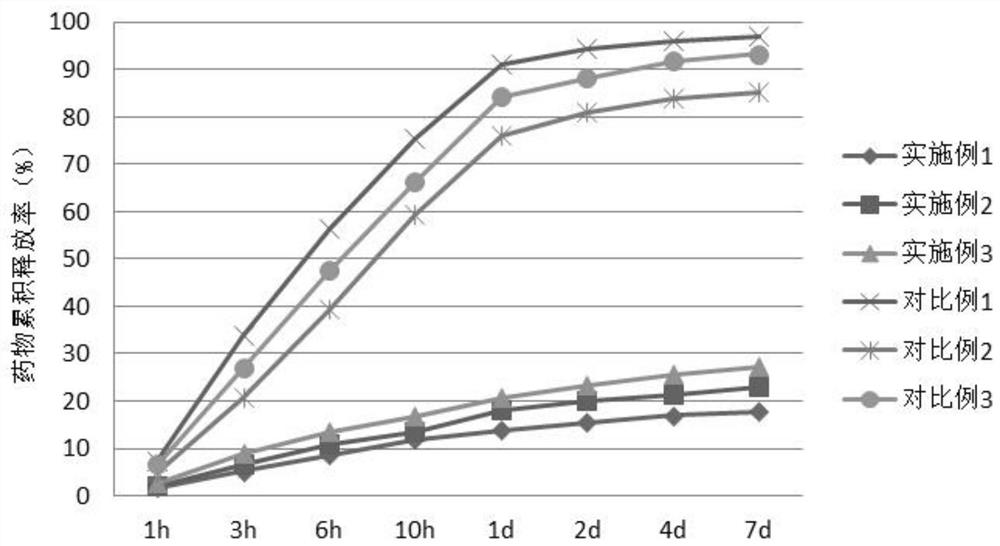
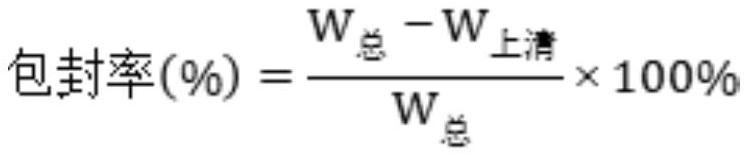A kind of polyglutamic acid graft metformin stereostructure nano-micelle and preparation method thereof
A technology of metformin and polyglutamic acid, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of short drug half-life and low encapsulation rate, and achieve improved encapsulation sealing rate, prolonging half-life, and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of polyglutamic acid grafted metformin stereotactic nano micelles, comprising the steps of:
[0029] (1) After cooling 1g of N-carboxy-L-glutamic acid-cyclic anhydride with liquid nitrogen, vacuumize it, and add 4ml of SOCl under an ice bath in a nitrogen atmosphere 2 , the acid halide reaction was 12h, and after the reaction was finished, the unreacted SOCl was distilled off under reduced pressure 2 , then add 35mlDMF, 8ml triethylamine and 0.7g metformin to react at room temperature for 12h, the reaction product is filtered after precipitation treatment, and after drying, N-(N,N-dimethylcarbamoyl)carbamoyl)-N -Carboxyl-L-glutamic acid-cyclic anhydride;
[0030] (2) After cooling 1g of N-carboxy-D-glutamic acid-anhydride in the ring with liquid nitrogen, vacuumize it, and add 4ml of SOCl under ice bath in a nitrogen atmosphere 2 , the acid halide reaction was 12h, and after the reaction was finished, the unreacted SOCl was distilled off under red...
Embodiment 2
[0034]A preparation method of polyglutamic acid grafted metformin stereotactic nano micelles, comprising the steps of:
[0035] (1) Cool 1g of N-carboxy-L-glutamic acid-cyclic anhydride with liquid nitrogen and then vacuumize, add 3ml of SOCl under ice bath in nitrogen atmosphere 2 , acid halide reaction 4h, after the end of the reaction, the unreacted SOCl was distilled off under reduced pressure 2 , then added 20ml DMF, 5ml triethylamine and 0.5g metformin to react at room temperature for 4h, the reaction product was filtered after precipitation treatment, and dried to obtain N-(N,N-dimethylcarbamoyl)carbamoyl)- N-carboxy-L-glutamic acid-cyclic anhydride;
[0036] (2) After cooling 1g of N-carboxy-D-glutamic acid-anhydride in the ring with liquid nitrogen, vacuumize it, and add 3ml of SOCl under an ice bath in a nitrogen atmosphere 2 , acid halide reaction 4h, after the end of the reaction, the unreacted SOCl was distilled off under reduced pressure 2 , then added 20ml DM...
Embodiment 3
[0040] A preparation method of polyglutamic acid grafted metformin stereotactic nano micelles, comprising the steps of:
[0041] (1) After cooling 1g of N-carboxy-L-glutamic acid-cyclic anhydride with liquid nitrogen, vacuumize it, and add 5ml of SOCl under an ice bath in a nitrogen atmosphere 2 , the acid halide reaction was 24h, and after the reaction was finished, the unreacted SOCl was distilled off under reduced pressure 2 , then add 50mlDMF, 10ml triethylamine and 0.8g metformin to react at room temperature for 24h, the reaction product is filtered after precipitation treatment, and after drying, N-(N,N-dimethylcarbamoyl)carbamoyl)-N -Carboxyl-L-glutamic acid-cyclic anhydride;
[0042] (2) After cooling 1g of N-carboxy-D-glutamic acid-anhydride in the ring with liquid nitrogen, vacuumize it, and add 5ml of SOCl under an ice bath in a nitrogen atmosphere 2 , the acid halide reaction was 24h, and after the reaction was finished, the unreacted SOCl was distilled off under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com