Multiple-target-point treating micelle for regulating and controlling microenvironment of Alzheimer disease (AD) and preparation method of multiple-target-point treating micelle
A multi-target, micellar technology, applied in the direction of non-active ingredients medical preparations, pharmaceutical formulas, nervous system diseases, etc., can solve the problem of lack of AD microenvironment adjustment, achieve stable blood circulation, simple and feasible preparation process , increase the effect of accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
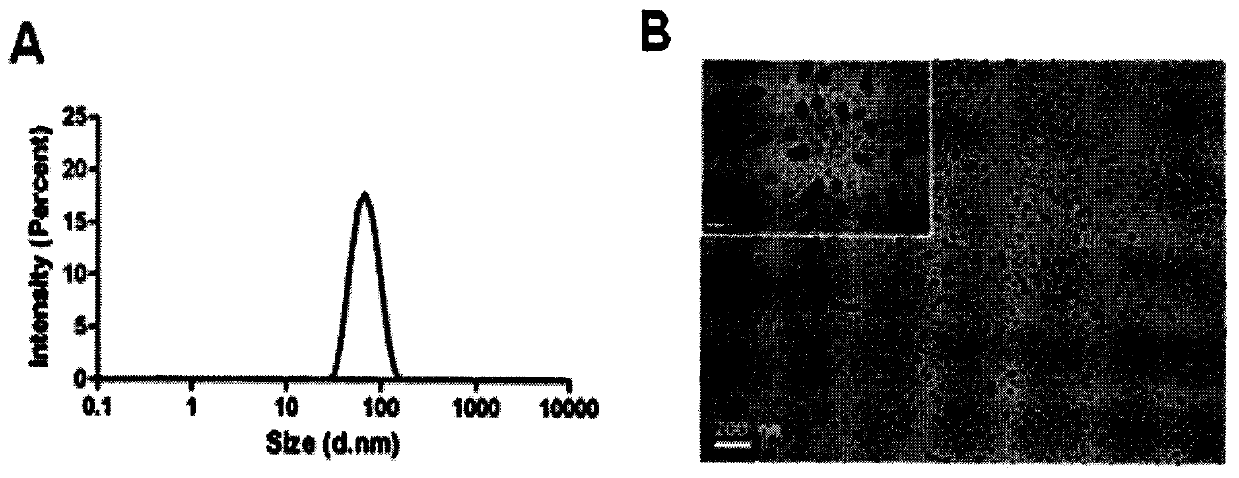
Image
Examples
Embodiment 1
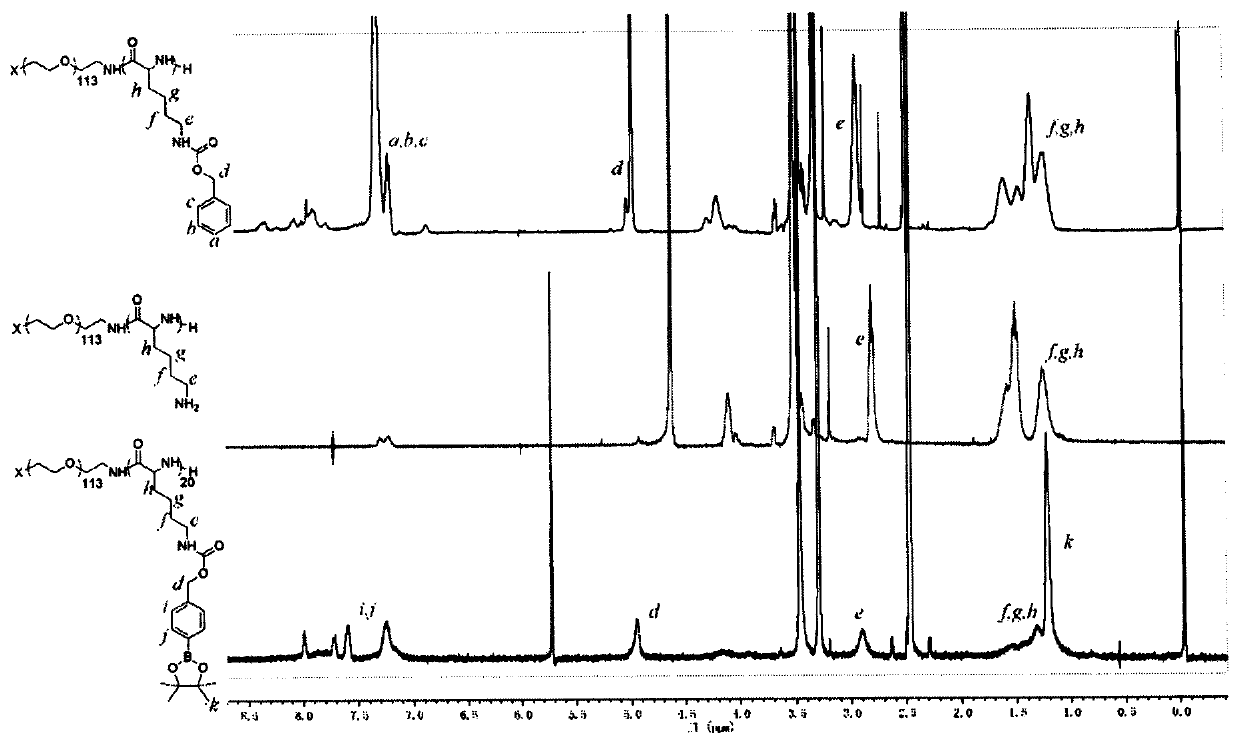
[0036] Ab peptide-modified polymer micellar nanoparticles loaded with insoluble AD treatment drug curcumin. Drug curcumin has been reported to have various mechanisms of action such as anti-inflammation and anti-oxidation, and is suitable for multi-target therapy of AD. Its polymer Synthetic methods such as figure 1 shown, including the following steps:
[0037] 1) Synthesis of Ab peptide-modified polyethylene glycol polylysine phenylboronate derivatives
[0038] (1) Weigh N 6 -Benzyloxycarbonyl-L-lysine 1g, triphosgene 0.3g, dissolved in 10mL tetrahydrofuran, under nitrogen protection, reacted at 50°C for 5 hours, precipitated with 50mL anhydrous n-hexane, and dried by suction to obtain lysine monomer;
[0039] (2) Weigh 210 mg of lysine monomer obtained in step (1), 100 mg of polyethylene glycol, dissolve in 3 mL of anhydrous N'N-dimethylformamide, and react at 55 ° C for 24 hours under nitrogen protection , use 30mL of anhydrous ether to precipitate, and filter and dry ...
Embodiment 2
[0048] Example 2 Ab peptide-modified polymer micelle nanoparticle preparation loaded with near-infrared probes
[0049] 1) Synthesis of Ab peptide-modified polyethylene glycol polylysine phenylboronate derivatives
[0050] (1) Weigh N 6 -Benzyloxycarbonyl-L-lysine 1g, triphosgene 0.45g, dissolved in 10mL tetrahydrofuran, under nitrogen protection, reacted at 55°C for 8 hours, precipitated with 80mL anhydrous n-hexane, and dried by suction to obtain lysine monomer;
[0051] (2) Weigh 240 mg of lysine monomer obtained in step (1), 100 mg of polyethylene glycol, dissolve in 3 mL of anhydrous N'N-dimethylformamide, and react at 60 ° C for 48 hours under nitrogen protection , use 30mL of anhydrous ether to precipitate, and filter and dry to obtain a white solid, dissolve 100mg of the white solid in a mixture of 1mL trifluoroacetic acid and 0.05mL hydrobromic acid (containing 30% acetic acid), react at room temperature for 6 hours, use pure water dialyzed and lyophilized;
[005...
Embodiment 3
[0059] Example 3 Ab peptide-modified polymer micelle nanoparticle formulation loaded with curcumin
[0060] 1) Synthesis of Ab peptide-modified polyethylene glycol polylysine phenylboronate derivatives
[0061] (1) Weigh N 6 -Benzyloxycarbonyl-L-lysine 1g, triphosgene 0.5g, dissolved in 10mL tetrahydrofuran, under nitrogen protection, reacted at 55°C for 10 hours, precipitated with 100mL anhydrous n-hexane, and dried by suction to obtain lysine monomer;
[0062] (2) Weigh 270 mg of lysine monomer obtained in step (1), 100 mg of polyethylene glycol, dissolve in 3 mL of anhydrous N'N-dimethylformamide, and react at 65 ° C for 72 hours under nitrogen protection , precipitated with 30 mL of anhydrous ether, and dried by suction to obtain a white solid. Dissolve 100 mg of the white solid in a mixture of 1 mL of trifluoroacetic acid and 0.03 mL of hydrobromic acid (containing 30% acetic acid), react at room temperature for 8 hours, dialyze with pure water and freeze-dry;
[0063...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com