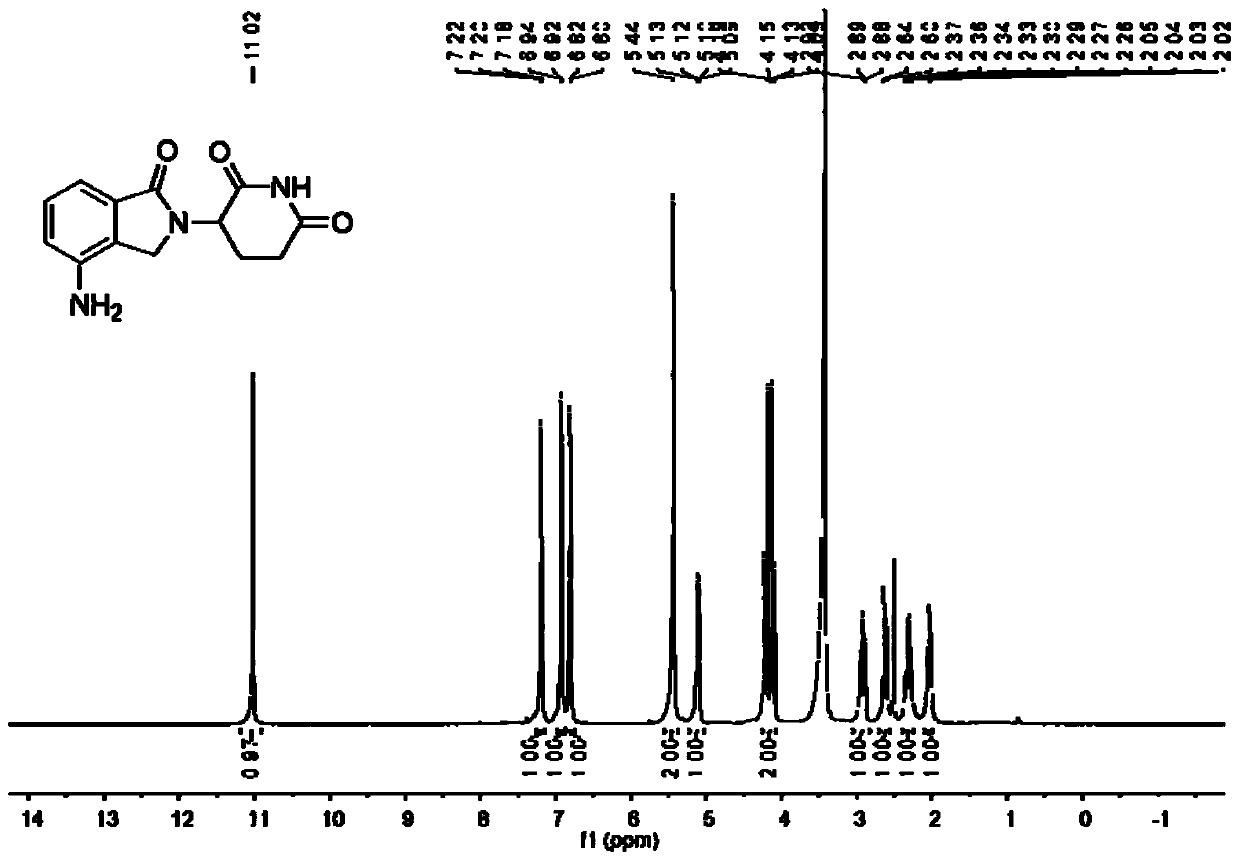
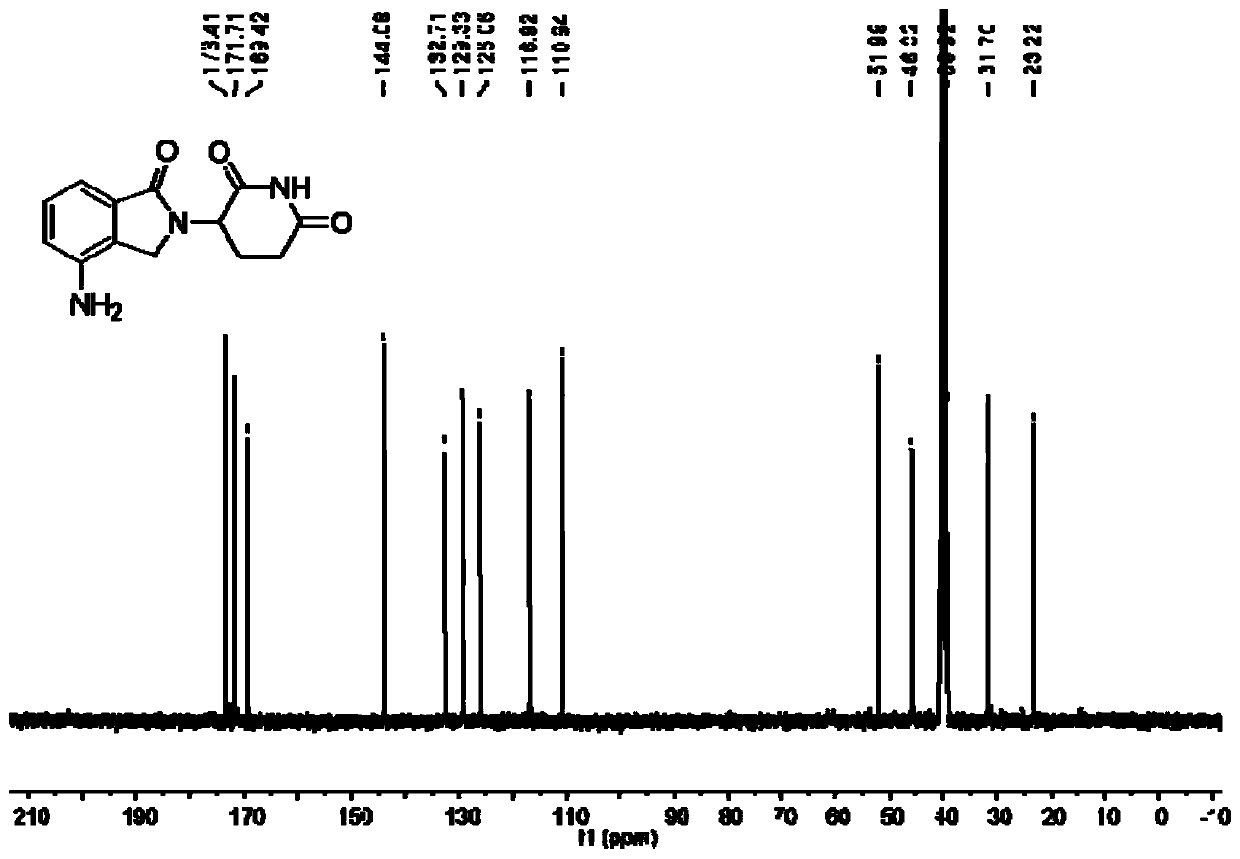
Method for synthesizing lenalidomide
A technology of lenalidomide and a synthesis method, applied in the field of synthesis of lenalidomide, can solve the problems of expensive raw materials, unsuitable for industrial production, difficult purification, etc., achieves improvement of quality and yield, and realizes green production and technology The effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: (1) the preparation of 2-bromomethyl-3-nitrobenzoic acid methyl ester: add 19.5g2-methyl-3-nitrobenzoic acid methyl ester in 200mL ethyl acetate ethyl ester, 18.6g N - 0.24 g of bromosuccinimide and benzoyl peroxide, the mixed solution was stirred at 65° C. for 1 hour, and then cooled to room temperature. 200 mL of water was added, the organic layer was separated and evaporated under vacuum to give a solid, which was recrystallized from petroleum ether to give the product. Pale yellow solid, 26.5g, yield 97%. The product was detected: m.p.: 69-70°C; 1HNMR (400MHz, CDCl3) δ8.11(d, J=7.8Hz, 1H), 7.96(d, J=7.9Hz, 1H), 7.55(t, J=8.0 Hz, 1H), 5.16(s, 2H), 4.00(s, 3H).
[0025] (2) Preparation of 3-(4-nitro-1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione: 10.9g 2-(bromomethyl )-Methyl 3-nitrobenzoate, 6.6g of 3-aminopiperidine-2,6-dione hydrochloride, and 5.96g of triethylamine were stirred at 85°C for 45 minutes under nitrogen protection. After the reac...
Embodiment 2
[0027] Embodiment 2: (1) Preparation of 2-bromomethyl-3-nitrobenzoic acid methyl ester: in 2L 1,2-ethylene dichloride, add 195.3g 2-methyl-3-nitrobenzoic acid methyl Esters, 186.1 g of N-bromosuccinimide and 2.46 g of benzoyl peroxide, the mixed solution was stirred at 85° C. for 1 hour, and then cooled to room temperature. 2 L of water was added, the organic layer was separated and evaporated under vacuum to give a solid which was recrystallized from petroleum ether to give the product. Pale yellow solid, 268.3g, yield 98%. The product was detected: m.p.: 68-70°C; 1H NMR (400MHz, CDCl3) δ8.10(d, J=7.8Hz, 1H), 7.94(d, J=7.9Hz, 1H), 7.55(t, J= 8.0Hz, 1H), 5.16(s, 2H), 4.00(s, 3H).
[0028] (2) Preparation of 3-(4-nitro-1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione: 108.8g 2-(bromomethyl )-Methyl 3-nitrobenzoate, 66.3g of 3-aminopiperidine-2,6-dione hydrochloride, and 596.3g of sodium carbonate were stirred at 100°C for 45 minutes under nitrogen protection. After the r...
Embodiment 3
[0030] Example 3: Step (1) in Example 3 is the same as step (3), the only difference is that 3-(4-nitro-1-oxo-1,3-dihydroiso Preparation of indol-2-yl)piperidine-2,6-dione: 4.7g methyl 2-(bromomethyl)-3-nitrobenzoate, 2.9g 3-aminopiperidine-2,6-dione A mixture of ketone hydrochloride and 2.5 g of triethylamine was stirred at 60° C. for 45 minutes under nitrogen protection. After the reaction was complete, the mixture was cooled to room temperature, 40 mL of ethanol was added, and stirred for 5 minutes. The precipitate was then collected by filtration and dried to yield the product. Off-white solid, 5.2g, yield 89%. The product was detected: m.p.: 274-275°C; 1H NMR (400MHz, DMSO) δ11.06(s, 1H), 8.48(d, J=8.0Hz, 1H), 8.19(d, J=7.3Hz, 1H) ,7.87(t,J=7.8Hz,1H),5.16(dd,J=13.0,4.8Hz,1H),4.87(dd,J=46.3,19.4Hz,2H),2.98–2.83(m,1H), 2.65–2.53(m,2H), 2.07–2.00(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com