A rare earth nanomaterial containing polystyrene shell and its biocoupling and application
A technology of rare earth nanomaterials and polystyrene, which is applied in nanotechnology for materials and surface science, luminescent materials, analytical materials, etc., can solve problems such as difficult to guarantee stability, weak covalent interaction, and antibody shedding, and achieve The effect of strong connection stability and good material dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
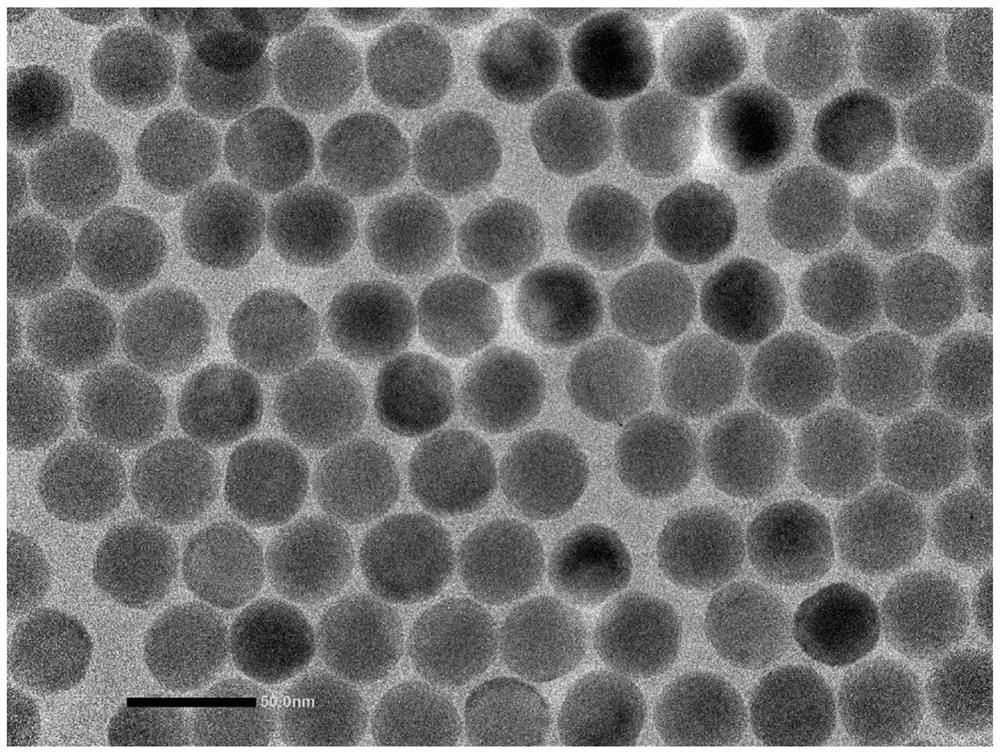
[0082] Example 1: β-NaYF 4 Synthesis and Luminescent Properties of :Yb,Er Cores
[0083] YCl 3 0.78mmol, YbCl 3 0.2mmol, ErCl 3 0.02mmol, OA (oleic acid oleic acid) 6mL and ODE (1-Octadecene octadecene) 15mL were added to a 100mL round bottom flask, heated to 150°C under vacuum until all the rare earth chlorides were dissolved, then cooled naturally to 60°C.
[0084] Add 2.5 mmol NaOH and 4 mmol NH dropwise to the cooled system 4 F in methanol solution 10mL, stirred for 30 minutes. After stirring, the system was heated to 150°C, and nitrogen was blown for 1 hour to remove methanol.
[0085] Under the protection of nitrogen, the reaction system was heated to 300°C and reacted for 1 h; after the reaction was completed, it was naturally cooled to room temperature.
[0086] Add 10mL of ethanol to the reaction product, centrifuge at 15000rpm for 10min, discard the supernatant solution; disperse the obtained solid in 10mL of cyclohexane, add 20mL of ethanol, and centrifuge ...
Embodiment 2
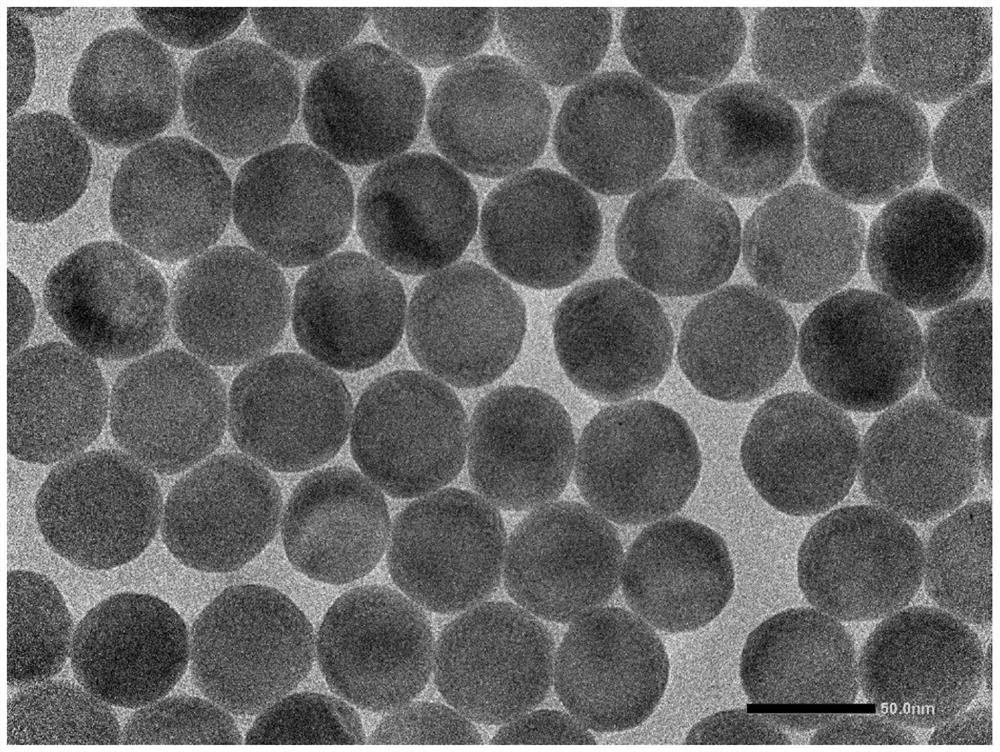
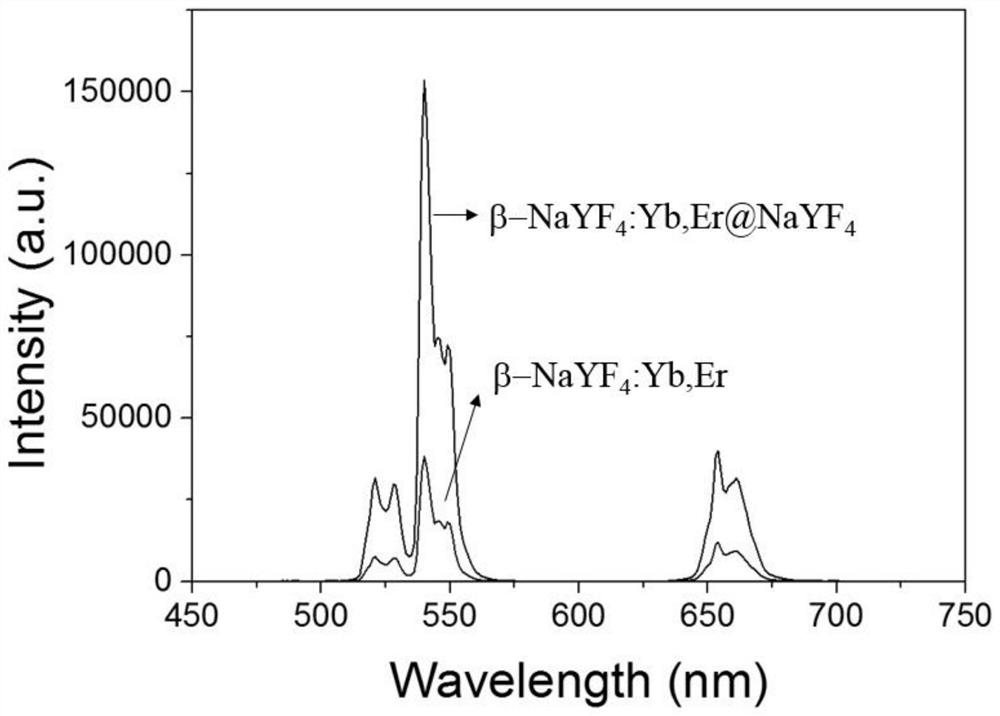
[0089] Example 2: β-NaYF 4 :Yb,Er@NaLuF 4 Synthesis of inner core and study of its luminescent properties
[0090] LuCl 3 Add 1mmol, 6mL of OA and 15mL of ODE into a 100mL round bottom flask, heat to 150°C under vacuum until all the rare earth chlorides are dissolved, and cool naturally to 90°C.
[0091] Add 200mgβ-NaYF to the cooled system 4 : Yb, Er core (embodiment 1) cyclohexane solution 10mL, heated to 150 ° C, nitrogen 1h to remove cyclohexane.
[0092] After cyclohexane was removed, it was naturally cooled to 60°C, and a solution containing 2.5mmol NaOH and 4mmol NH was added dropwise to the cooled system. 4 F in methanol solution 10mL, stirred for 30 minutes. After stirring, the system was heated to 150°C, and nitrogen was blown for 1 hour to remove methanol.
[0093] Under the protection of nitrogen, the reaction system was heated to 300° C. for 1 h. After the reaction was completed, it was naturally cooled to room temperature.
[0094] Add 10mL ethanol to the r...
Embodiment 3
[0097] Example 3: α-NaYF 4 :Synthesis of Yb, Nd Core and Study on Luminescent Properties
[0098] Take 2.84g (10mmol) oleic acid, 2.67g (10mmol) oleylamine, 5.04g (20mmol) octadecene, 1mmol rare earth trifluoroacetate (Y:Yb:Nd=33:7:60), 1mmol trifluoro Sodium acetate was placed in a 100ml three-neck bottle, and the system was sealed, pumped and heated to 110°C until a transparent and clear solution was formed.
[0099] Pump and exchange air three times, blow nitrogen, heat up to 300°C and react for 30 minutes; after the reaction, cool naturally, wash the three-necked flask with 3mL cyclohexane, add 10mL absolute ethanol, centrifuge at 15000rpm for 10min, and use about 20ml ethanol / cyclohexane for precipitation. Hexane mixed solution (ethanol / cyclohexane volume ratio 3 / 1) was washed three times, and the obtained α-NaYF 4 : Yb, Nd core stored in 10ml cyclohexane.
[0100] to α-NaYF 4 : Yb, Nd inner core was characterized by transmission electron microscopy, the results are a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com