Aspirin-contained enteric capsules and preparation method thereof
A technology of aspirin and enteric-coated capsules, which is applied in the field of aspirin preparations, can solve the problems of aggregation of pellets, affecting the dissolution and release of aspirin, and failure to achieve drug efficacy, and achieve the effect of preventing pellets from sticking together
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
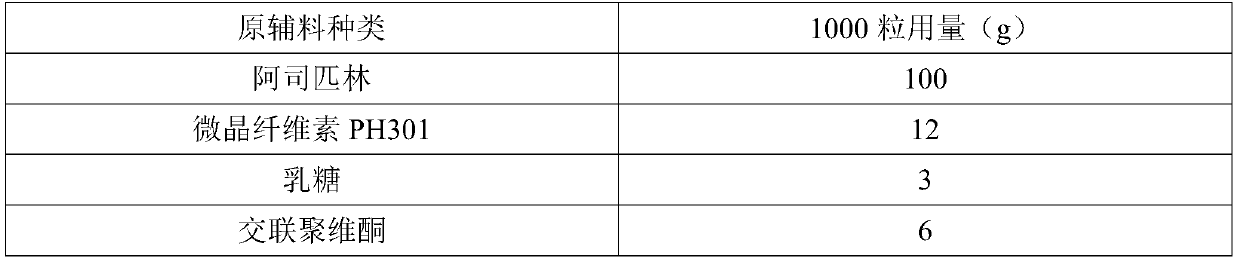
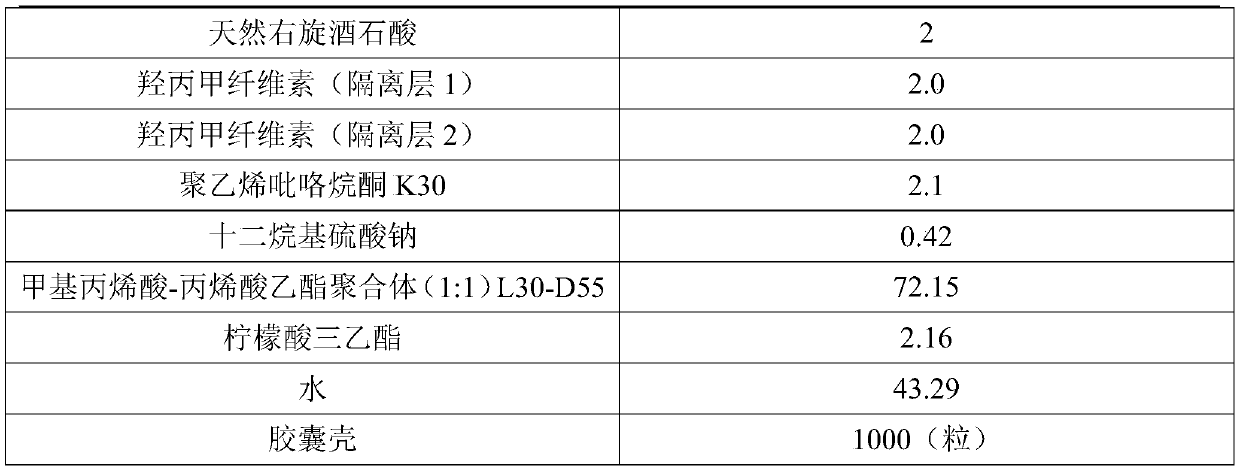
[0040] Embodiment 1: preparation embodiment (outer insulation layer 2g, thicker)
[0041]
[0042]
[0043] Preparation process: 1. Raw and auxiliary materials are sieved for later use.
[0044] 2. For adhesive preparation, add polyvinylpyrrolidone k30 into water, stir until completely dissolved, add malic acid into the above solution, and continue stirring until completely dissolved.
[0045] 3. Total mixing Add aspirin, microcrystalline cellulose, lactose, and cross-linked polyvinylpyrrolidone in sequence to the wet granulation mixer for mixing.
[0046] 4. Preparation of soft material Add the binder solution to the total blended powder, stir at 100 rpm, shear at 1200 rpm, and run for 3 minutes to obtain a soft material for use.
[0047] 5. Pelletizing The prepared soft material is extruded by an extrusion and spheronization machine, and the extrusion frequency is 30HZ
[0048] Cut and round the obtained strips at a frequency of 20HZ for 1min
[0049] 15HZ 1min.
...
Embodiment 2
[0055] Embodiment 2: preparation embodiment (no outer barrier layer, promptly without barrier layer 2)
[0056]
[0057]
[0058] Preparation Process:
[0059] 1. Raw and auxiliary materials are sieved for later use.
[0060] 2. For adhesive preparation, add polyvinylpyrrolidone k30 into water, stir until completely dissolved, add malic acid into the above solution, and continue stirring until completely dissolved.
[0061] 3. Total mixing Add aspirin, microcrystalline cellulose, lactose, and cross-linked polyvinylpyrrolidone in sequence to the wet granulation mixer for mixing.
[0062] 4. Preparation of soft material Add the binder solution to the total blended powder, stir at 100 rpm, shear at 1200 rpm, and run for 3 minutes to obtain a soft material for use.
[0063] 5. Pelletizing The prepared soft material is extruded by an extrusion and spheronization machine, and the extrusion frequency is 30HZ
[0064] Cut and round the obtained strips at a frequency of 20HZ f...
Embodiment 3
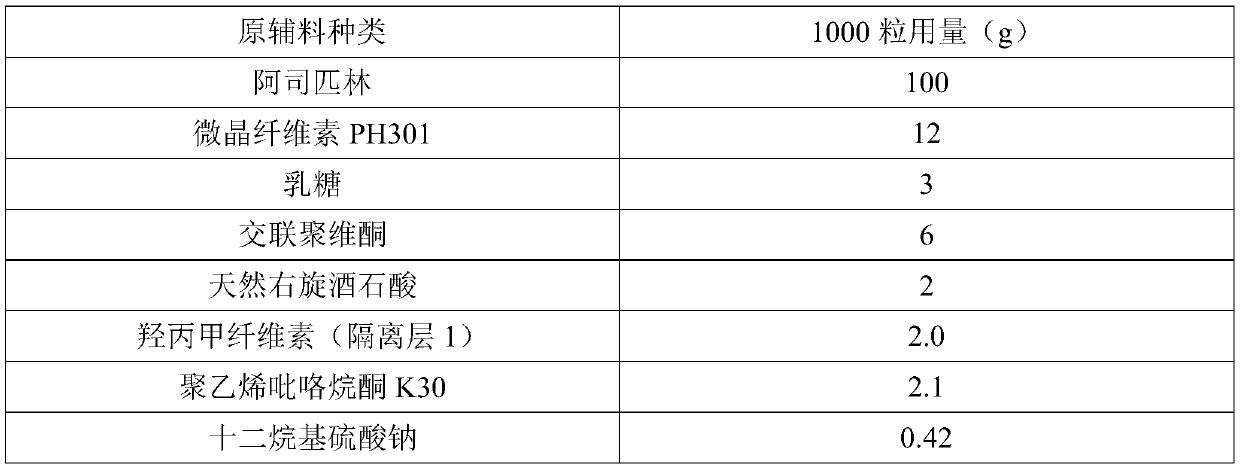
[0071] Preparation Example (best ratio, 2.5g of isolation layer)
[0072] Types of raw materials Dosage for 1000 capsules (g) aspirin 100 Microcrystalline Cellulose PH301 12 lactose 3 Crospovidone 6 natural d-tartaric acid 2 Hypromellose (isolation layer 1) 2.0 Hypromellose (isolation layer 2) 0.5 Polyvinylpyrrolidone K30 2.1 Sodium dodecyl sulfate 0.42 Methacrylic acid-ethyl acrylate polymer (1:1) L30-D55 72.15 triethyl citrate 2.16 water 43.29 capsule shell 1000(grains)
[0073] Preparation Process:
[0074] 1. Raw and auxiliary materials are sieved for later use.
[0075] 2. For adhesive preparation, add polyvinylpyrrolidone k30 into water, stir until completely dissolved, add malic acid into the above solution, and continue stirring until completely dissolved.
[0076] 3. Total mixing Add aspirin, microcrystalline cellulose, lactose, and cross-linked polyvinylpyrrolidone in seque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com