Chemiluminiscence quantitative detection for excrement calprotectin, and detection method and application thereof in intestinal health detection
A technology for quantitative detection of calprotectin, applied in chemiluminescence/bioluminescence, analysis through chemical reactions of materials, measurement devices, etc., can solve problems such as intolerance of colonoscopy pain, low detection sensitivity, and high cost. Achieve high clinical auxiliary value, simple detection steps, and good consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0046] Embodiment scheme one, "streptavidin-biotin" system;
[0047] A calprotectin detection reagent, comprising:
[0048] Biotin-labeled calprotectin monoclonal or polyclonal antibody;
[0049] Marker-labeled calprotectin monoclonal or polyclonal antibody, as an example, the luminescent signal marker can be acridine lipid, HRP, alkaline phosphatase or luminal sodium;
[0050] Calprotectin calibrator, the components include: human fecal calprotectin antigen;
[0051] Calprotectin control product, the ingredients include: human fecal calprotectin antigen;
[0052] Sample to be tested;
[0053] Streptavidin-coated magnetic particles;
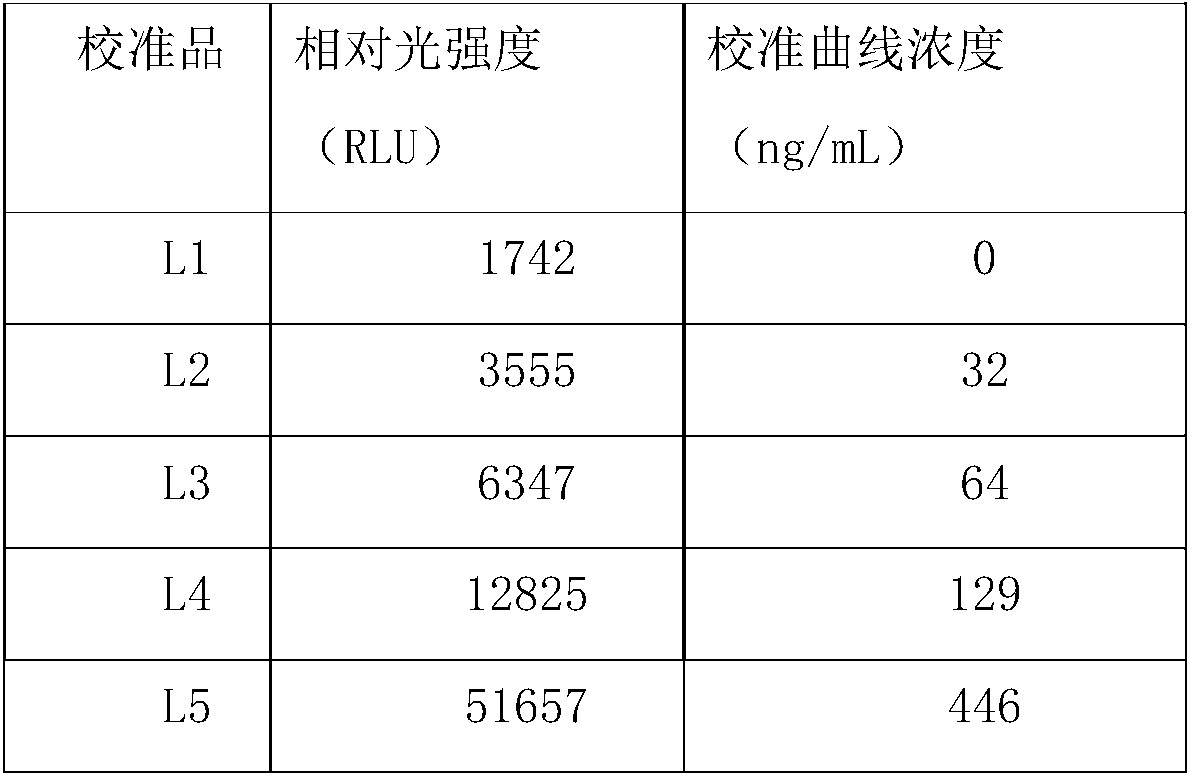
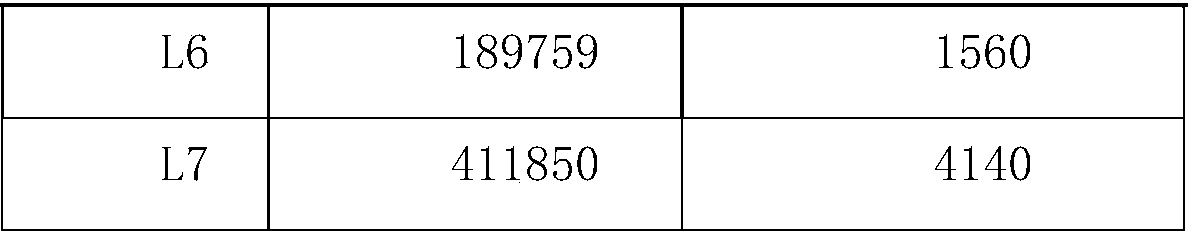
[0054] Calprotectin calibration card: burn relevant information of calprotectin calibration products in the card;
[0055] Calprotectin reagent card: burn relevant information of calprotectin calibration curve in the card;
[0056] Cleaning solution, the composition of the cleaning solution includes: 0.01mol / L-0.2mol / L phosphate, 0.5mol / L-2...
Embodiment approach 2
[0060] Embodiment 2, the magnetic particle system directly coated with antibodies;
[0061] A chemiluminescence quantitative detection of fecal calprotectin comprising:
[0062] Magnetic particles coated with monoclonal or polyclonal antibodies to calprotectin,
[0063] Marker-labeled calprotectin monoclonal or polyclonal antibody antibody, as an example, the marker can be acridine lipid, HRP, alkaline phosphatase or luminal sodium;
[0064] Calprotectin calibrator, the components include: human fecal calprotectin antigen;
[0065] Calprotectin control product, the ingredients include: human fecal calprotectin antigen;
[0066] Sample to be tested;
[0067] Calprotectin calibration card: burn relevant information of calprotectin calibration products in the card;
[0068] Calprotectin reagent card: burn relevant information of calprotectin calibration curve in the card;
[0069] Cleaning solution, the composition of the cleaning solution includes: 0.01mol / L-0.2mol / L phosph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com