Recombinant rhabditiform plasmid and application thereof to expression of PCV3 Cap protein and vaccine
A recombinant plasmid and rod-shaped technology, applied in the biological field, can solve the problems of difficult to estimate the impact of pig farming, no effective prevention and control measures for PCV3, etc., and achieve the effect of good antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 Amplification, cloning and identification analysis of PCV3 Cap gene
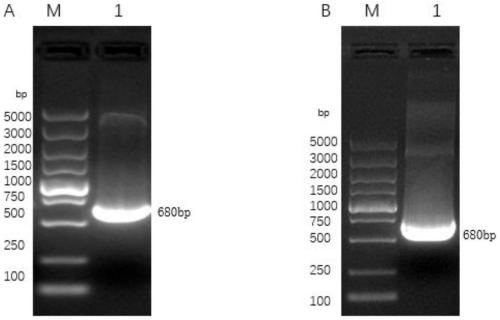
[0045] Using the plasmid containing the target gene PCV3 Cap as a template, two pairs of primers (Table 1) for amplifying the PCV3 Cap gene were used to amplify the target gene. 40s at ℃, 30s at 72℃, 5min at 72℃, 4℃∞, 35 cycles. PCV3-Cap2: 95°C for 5min, 95°C for 30s, 65°C for 25s, 72°C for 30s, 72°C for 5min, 4°C∞, 35 cycles. The target fragment was separated and recovered by 1% agarose gel electrophoresis, cloned into the pEASY-Blunt vector, and transformed into Trans5α competent cells, spread on a solid LB plate, cultured upside down at 37°C for 12 hours, and picked a single clone strain Shake culture in liquid LB for 12 hours, extract the plasmids and name them pEPC1 and pEPC2, and identify them by double-enzyme digestion with EcoRI and NotI, XhoI and KpnI respectively, and further confirm by Sanger sequencing.
[0046] Use primer shown in table 1 to carry out the amplification of P...
Embodiment 2
[0048] Construction and Identification of Embodiment 2 Recombinant Transfer Plasmid
[0049] The correctly sequenced pEPC1 and pEPC2 were digested with enzymes, respectively connected to the pFBD Dual vector, and named pFBD-P2C, the plasmids were extracted by conventional molecular biology methods, and identified by double digestion with EcoRI and NotI, XhoI and KpnI respectively.
[0050] The plasmids and expression vectors pFBD that were correctly identified by sequencing and containing PCV3-Cap1 and PCV3-Cap2 genes were digested with corresponding enzymes respectively, and connected to pFBD in sequence to construct a shuttle plasmid pFBD-P2C containing two PCV3Cap genes (such as Figure 4A shown). The enzyme digestion results were as follows: Figure 4B As shown, the plasmid pFBD-P2C can cut out a single gene fragment ( Figure 4B , lanes 2, 3) and a fragment containing two genes ( Figure 4A , lane 4), indicating that the plasmid was constructed successfully. DNA seque...
Embodiment 3
[0051] Example 3 Construction and Identification of Recombinant Bacmid-P2C
[0052] Transformation of plasmid pFBD-P2C into DH10Bac TM Competent cells, and spread on the blue-white spot screening plate containing tetracycline (10 μg / mL), kanamycin sulfate (50 μg / mL), gentamicin (7 μg / mL), IPTG / X-Gal, Inverted culture at 37°C for 48h. Pick the white colony and add it to the SOC medium, shake it for 3 hours, carry out PCR identification of the bacterial liquid according to the general primers shown in Table 1, and extract the correctly identified strain from the bacmid, and name it Bacmid-P2C.
[0053] Utilize the general primers in Table 1 to carry out PCR verification on the Bacmid-P2C bacterial solution, the results are as follows Figure 5 As shown, the primers Puc / M13-R and PHF were used to amplify to obtain a band of about 1400bp, and the primers Puc / M13-F and P10R were used to amplify to obtain a band of about 2400bp, which was consistent with the expected results, indi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com