3,7-disubstituted phenoxazine derivatives and preparation method thereof
A phenoxazine and disubstituted technology, which is applied in the field of 3,7-disubstituted phenoxazine derivatives and their preparation, can solve the problems of structural rigidity, limited application space, and inability to connect with other units to achieve nitration The effect of mild reaction conditions, simple synthesis method and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation method of 3,7-dinitro-N-(4-nitrophenyl) phenoxazine comprises the steps:
[0050] (1) Preparation of N-(4-nitrophenyl) phenoxazine:
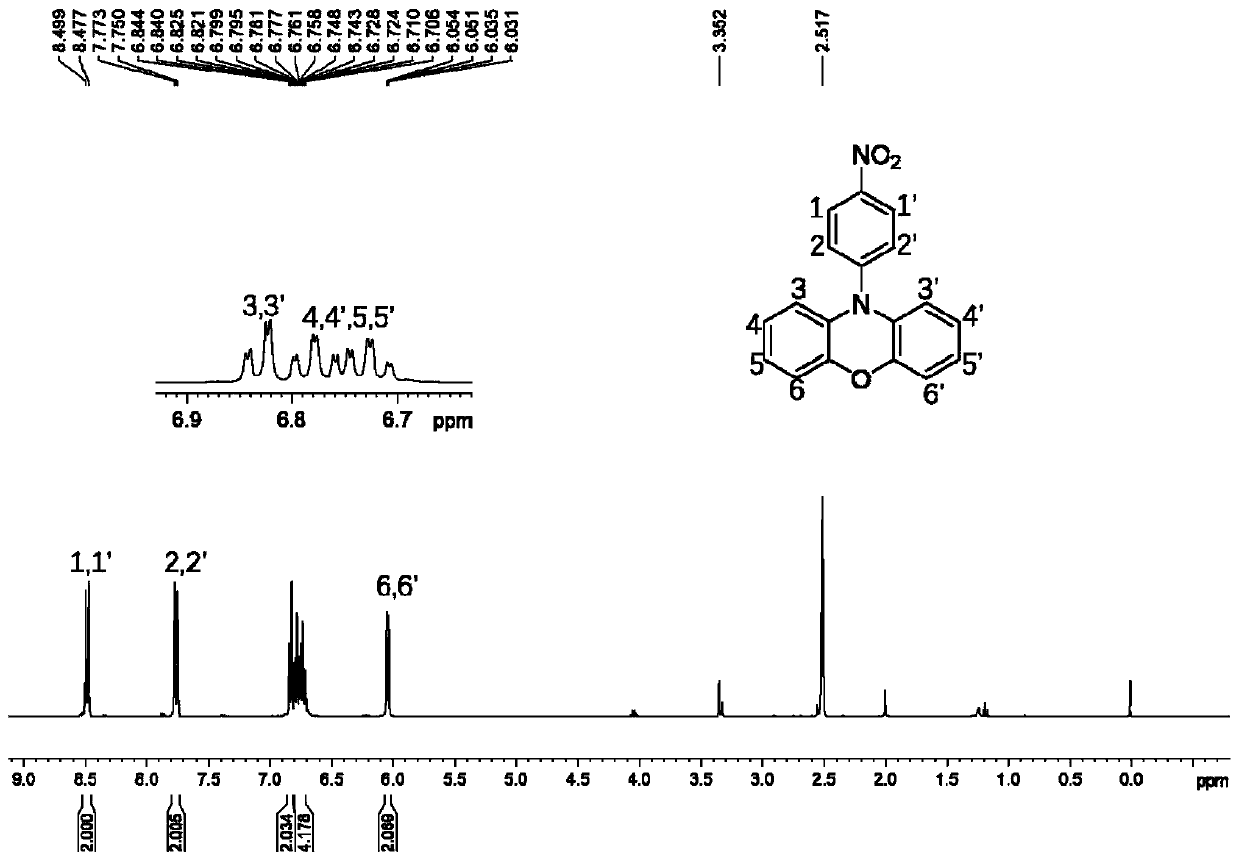
[0051] Weigh 16.4mmol of phenoxazine, 17.2mmol of 4-fluoronitrobenzene and 17.2mmol of cesium fluoride, dissolve them in 30mL of dry dimethyl sulfoxide, and heat and stir the mixture at 120°C for 18h in a nitrogen atmosphere , then cooled to room temperature, poured into a mixed solution of ethanol and deionized water, filtered, and washed with water to obtain N-(4-nitrophenyl)phenoxazine, a red solid, with a yield of 92%; 1 H-NMR (400MHz, DMSO, ppm): 8.499-8.477 (d, 2H), 7.773-7.750 (d, 2H), 6.844-6.706 (m, 6H), 6.054-6.031 (d, 2H);
[0052] The preparation process is as follows:
[0053]
[0054] The preparation method of 3,7-dinitro-N-(4-nitrophenyl) phenoxazine, the steps are as follows:
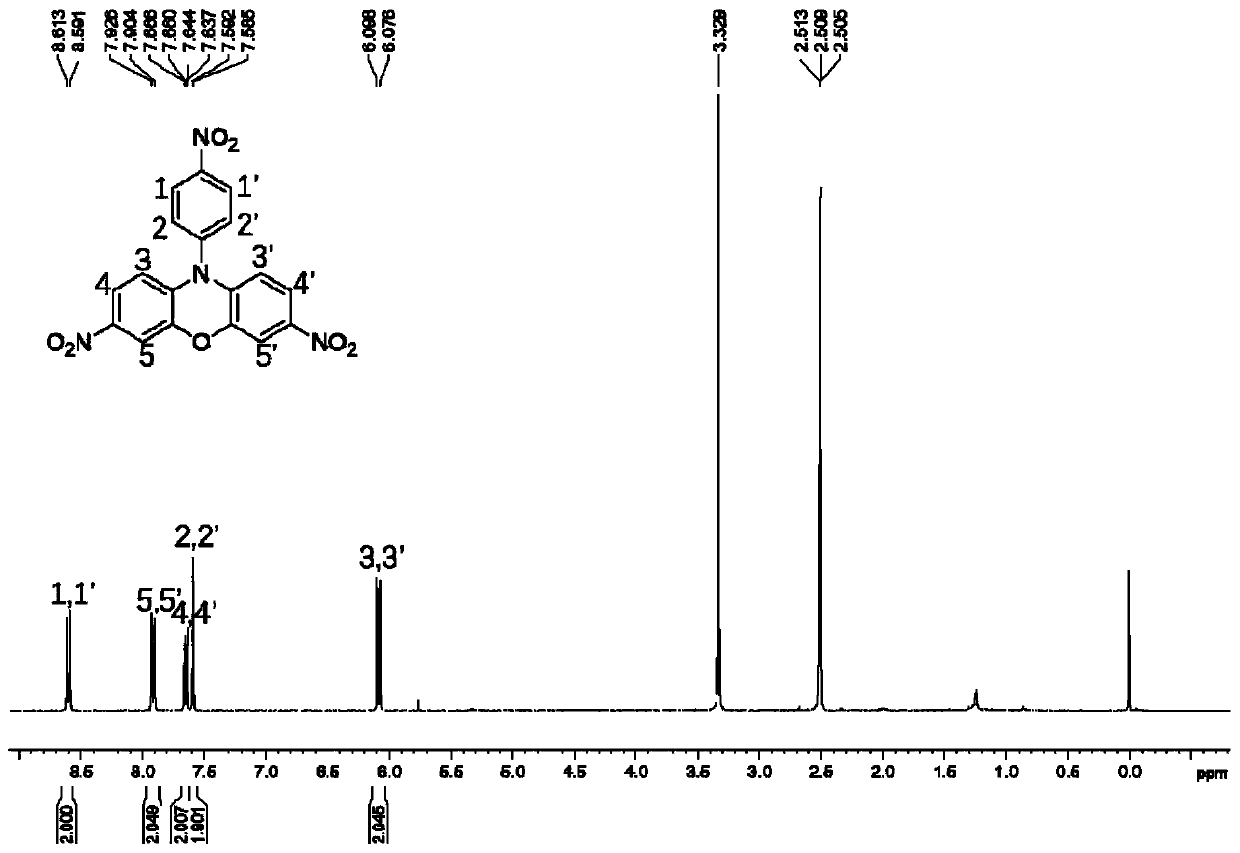
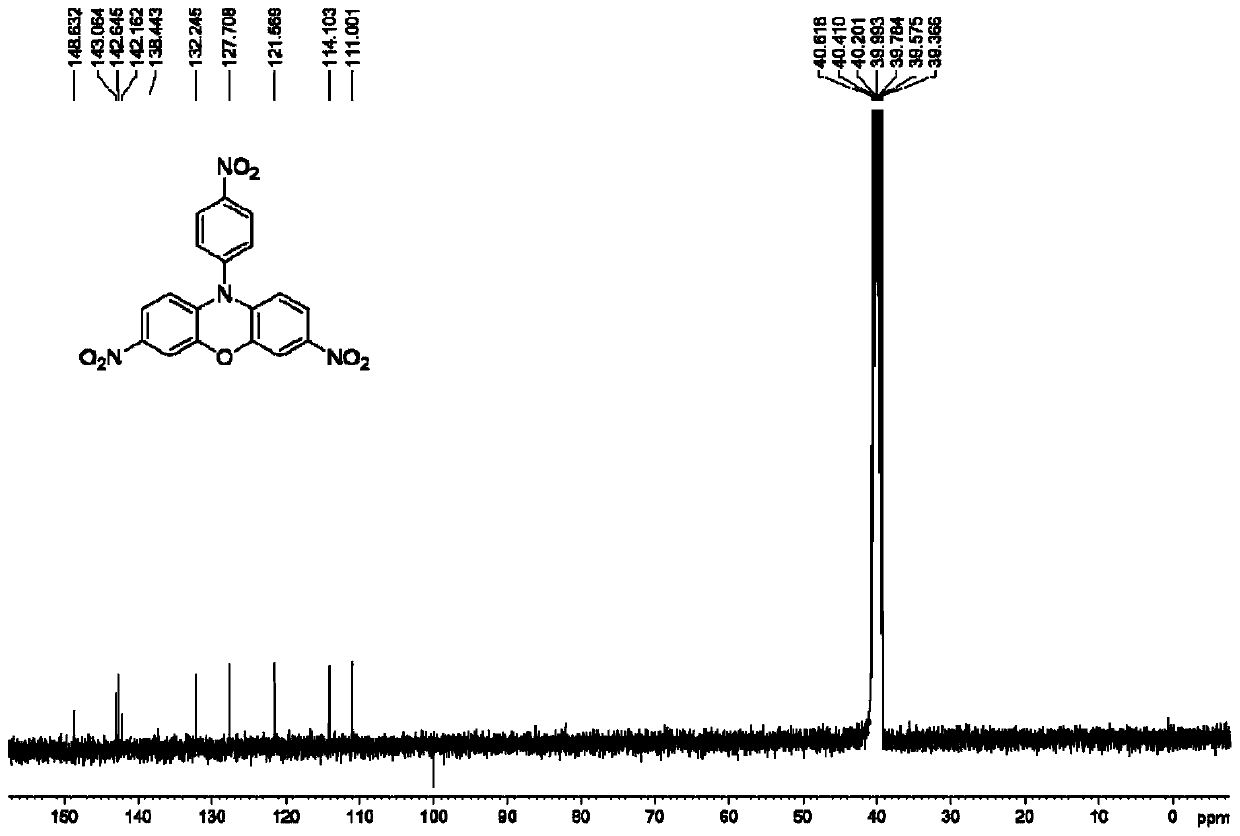
[0055] Weigh 6mmolCu(NO 3 ) 2 ·3H 2 O, measure 10 mL of acetic acid and 10 mL of acetic anhydride, stir and mix at room te...
Embodiment 2
[0059] The preparation method of 3,7-diamino-N-(4-aminophenyl) phenoxazine comprises the steps:
[0060] Weigh 2.54 mmol of 3,7-dinitro-N-(4-nitrophenyl) phenoxazine prepared in Example 2, 9.58 mmol of tin particles and 20 mL of concentrated hydrochloric acid with a mass concentration of 38% and put them into the reaction In the bottle, first reflux at 85°C for 12h in a nitrogen atmosphere, then reflux at 95°C for 12h, then cool to room temperature, add sodium hydroxide solution to adjust the pH to 7, stand still, filter and wash with water to obtain the crude product, and dissolve the crude product In tetrahydrofuran, petroleum ether was added thereto until a large amount of solids were precipitated to obtain 3,7-diamino-N-(4-aminophenyl)phenoxazine as an orange-red powder with a yield of 38.18%, in which 3, The yield of 7-dinitro-N-(4-nitrophenyl)phenoxazine to 3,7-diamino-N-(4-aminophenyl)phenoxazine can reach 83%; 1 H-NMR (400MHz, DMSO, ppm): 6.889-6.869 (d, 2H), 6.708-6....
Embodiment 3
[0064] The preparation method of 3,7-diacetyl-N-(4-formic acid methyl ester phenyl) phenoxazine comprises the steps:
[0065] (1) the preparation of N-(4-formic acid methyl phenyl) phenoxazine:
[0066] Take 5mmol of phenoxazine, 10mmol of methyl 4-iodobenzoate and 6mmol of potassium carbonate, dissolve them in 30mL of dry N,N-dimethylformamide, and stir the mixture in an oil bath at 220°C in a nitrogen atmosphere 6h, then cooled to room temperature, poured into dichloromethane, dissolved organic matter with dichloromethane, then filtered to remove inorganic matter, column chromatography (wherein the volume ratio of ethyl acetate to n-hexane was 1:10), to obtain N -(4-methyl formate phenyl)phenoxazine, light yellow crystalline solid, yield 94%; 1 H-NMR (400MHz, DMSO, ppm): 8.282-8.250 (d, 2H), 7.462-7.430 (d, 2H), 6.706-6.702 (d, 6H), 6.654-6.650 (d, 2H), 6.599-6.594 (d, 2H), 3.974(s, 3H), 5.934-5.911(2d, 2H);
[0067] The preparation process is as follows:
[0068]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com