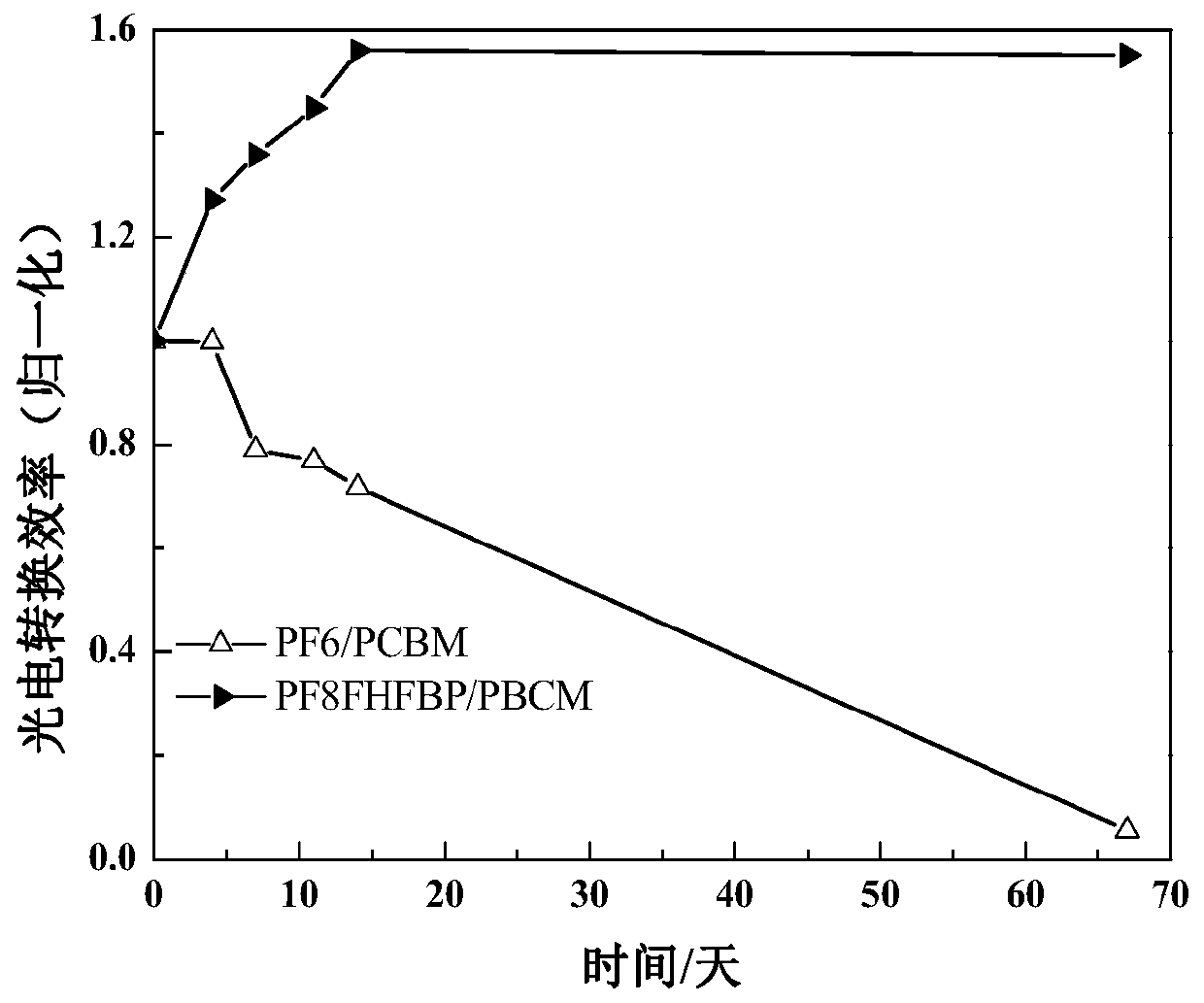
Electron transport layer for improving stability of reverse perovskite solar cell and preparation method
A technology of electron transport layer and solar cell, applied in the field of solar cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
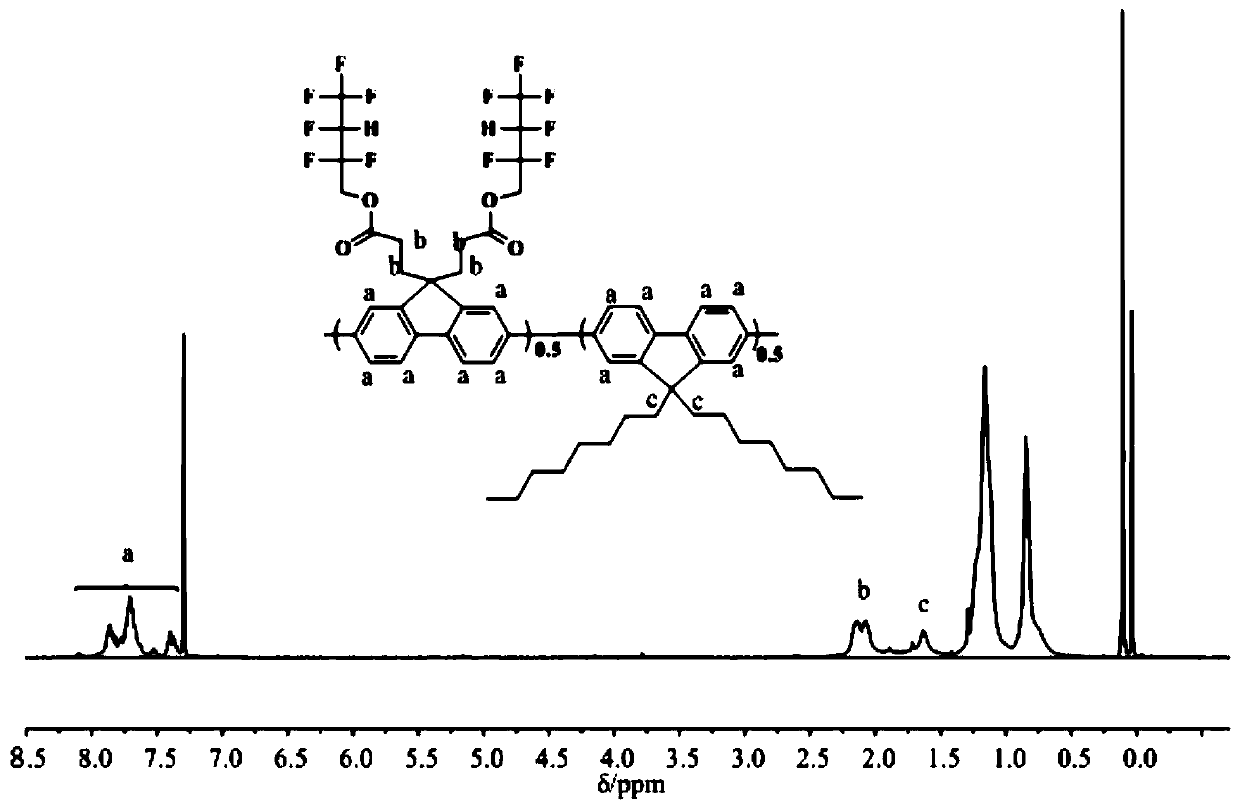
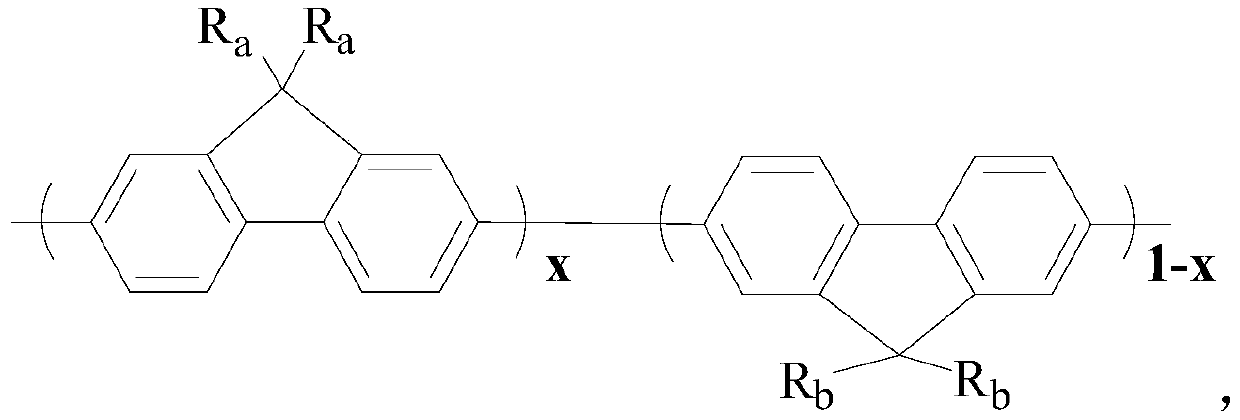
[0045] Preparation of fluorine-containing fluorene monomer, 2,7-dibromo-9,9-bis(hexafluorobutyl propionate)fluorene:
[0046] After placing a magnetic stirrer in a 100mL three-necked flask equipped with a thermometer, add 3.3g (10.2mmol) 2,7-dibromofluorene, 0.25g (0.78mmol) tetrabutylammonium bromide and 25mL toluene in sequence, and vacuumize Nitrogen was blown to maintain the nitrogen atmosphere, and then 5 mL of 50% potassium hydroxide aqueous solution was slowly added dropwise with a syringe. After magnetic stirring for about 30 minutes, use an ice-water bath, and after the temperature of the reaction system is constant, add 9.676 g (41 mmol) of hexafluorobutyl acrylate dropwise with a syringe. After the hexafluorobutyl acrylate was added dropwise, the stirring was continued for about 1 hour, and the temperature was raised to 25° C. for 6 hours. After the reaction, the reaction solution was poured into a separatory funnel, diluted with an appropriate amount of toluene, w...
Embodiment 2
[0048] Preparation of fluorine-containing fluorene monomer, 2,7-dibromo-9,9-bis(dodecafluoroheptyl propionate)fluorene:
[0049] After placing a magnetic stirrer in a 100mL three-neck flask equipped with a thermometer, add 3.3g (10.2mmol) 2,7-dibromofluorene, 0.16g (0.78mmol) tetraethylammonium bromide and 25mL dichloroethane , evacuated and ventilated with nitrogen to maintain the nitrogen atmosphere, and then slowly added 5 mL of 50% sodium hydroxide aqueous solution dropwise with a syringe. After magnetic stirring for about 30 minutes, use an ice-water bath, and after the temperature of the reaction system is constant, add 15.832 g (41 mmol) of dodecafluoroheptyl acrylate dropwise with a syringe. After the dodecafluoroheptyl acrylate was added dropwise, stirring was continued for about 1 h, and the temperature was raised to 30° C. for 8 h. After the reaction, pour the reaction solution into a separatory funnel, add an appropriate amount of dichloroethane to dilute, wash an...
Embodiment 3
[0051] Preparation of fluorine-containing fluorene monomer, 2,7-dibromo-9,9-di(tridecafluorooctyl propionate) fluorene:
[0052] After placing a magnetic stirrer in a 100mL three-neck flask equipped with a thermometer, add 3.3g (10.2mmol) 2,7-dibromofluorene, 0.21g (0.78mmol) tetrapropylammonium bromide and 25mL chloroform in sequence, and vacuumize Nitrogen was blown to maintain the nitrogen atmosphere, and then 5 mL of 50% potassium hydroxide aqueous solution was slowly added dropwise with a syringe. After magnetic stirring for about 30 minutes, use an ice-water bath, and after the temperature of the reaction system is constant, add 17.138 g (41 mmol) of trifluorooctyl acrylate dropwise with a syringe. After the trifluorooctyl acrylate was added dropwise, the stirring was continued for about 1 h, and the temperature was raised to 30° C. for 10 h. After the reaction, the reaction solution was poured into a separatory funnel, diluted with an appropriate amount of chloroform, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com