Method for selectively synthesizing polysubstituted dihydroquinazolinone or quinazolinone
A technology of dihydroquinazolinone and quinazolinone is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry and other directions, and can solve problems such as poor stability and operability, The problems such as non-recyclable and recycled use, and the catalyst is not easy to recover, to achieve the effect of convenient large-scale production, simple synthesis method and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 uses isatoic anhydride, aniline and benzaldehyde as raw materials to synthesize 2,3-diphenyl-2,3-dihydroquinazolin-4 (1H)-ones using different kinds of heteropolyacid ionic liquids For comparative experiments, the experiments were divided into eight groups, and each group was added with different kinds of heteropolyacid ionic liquids
[0030] Group A: [MIMPS] 3 PMo 12 o 40
[0031] Group B: [MIMPS] 3 PW 12 o 40
[0032] Group C: [PyPS] 3 PMo 12 o 40
[0033] Group D: [PyPS] 3 PW12O 40
[0034] Group E: [TEAPS] 3 PMo 12 o 40
[0035] Group F: [TEAPS] 3 PW 12 o40
[0036] Group G: H 3 PW 12 o 40
[0037] Group H: PyPSCl
[0038] The specific experimental method is as follows:
[0039] (1) 2mmol of isatoic anhydride, 2.5mmol of aniline, 2.5mmol of benzaldehyde and 0.04mmol of the ionic liquid of the A-G group or 0.12mmol of the ionic liquid of the H group are sequentially added to the reactor, stirred and mixed evenly;
[0040] (2) T...
Embodiment 2
[0044] Example 2 Using isatoic anhydride, aniline and benzaldehyde as raw material to synthesize 2,3-diphenyl-2,3-dihydroquinazolin-4(1H)-ketone using different amounts of [PyPS] 3 PW 12 o 40 The comparative experiment, the experiment is divided into six groups, each group added different amounts of [PyPS] 3 PW 12 o 40
[0045] Group A: 1mol%, [PyPS] 3 PW 12 o 40
[0046] Panel B: 2mol%, [PyPS] 3 PW 12 o 40
[0047] Panel C: 3mol%, [PyPS] 3 PW 12 o 40
[0048] Panel D: 4mol%, [PyPS] 3 PW 12 o 40
[0049] Group E: 5mol%, [PyPS] 3 PW 12 o 40
[0050] Group F: 6mol%, [PyPS] 3 PW 12 o 40
[0051] The specific experimental method is as follows:
[0052] (1) Add 2mmol of isatoic anhydride, 2.5mmol of aniline, 2.5mmol of benzaldehyde and the heteropolyacid ionic liquids of A-F groups respectively into the reactor, stir and mix evenly;
[0053] (2) The reactor was heated by microwave heating to 90° C. to start the reaction, and after 0.5 h of reaction, the...
Embodiment 3
[0058] (1) Mix isatoic anhydride 2mmol, aniline 2.5mmol, benzaldehyde 2.5mmol and [PyPS] 3 PW 12 o 40 Add 0.04mmol to the reactor in turn, stir and mix evenly;
[0059] (2) add hydrogen peroxide 3.0mmol;
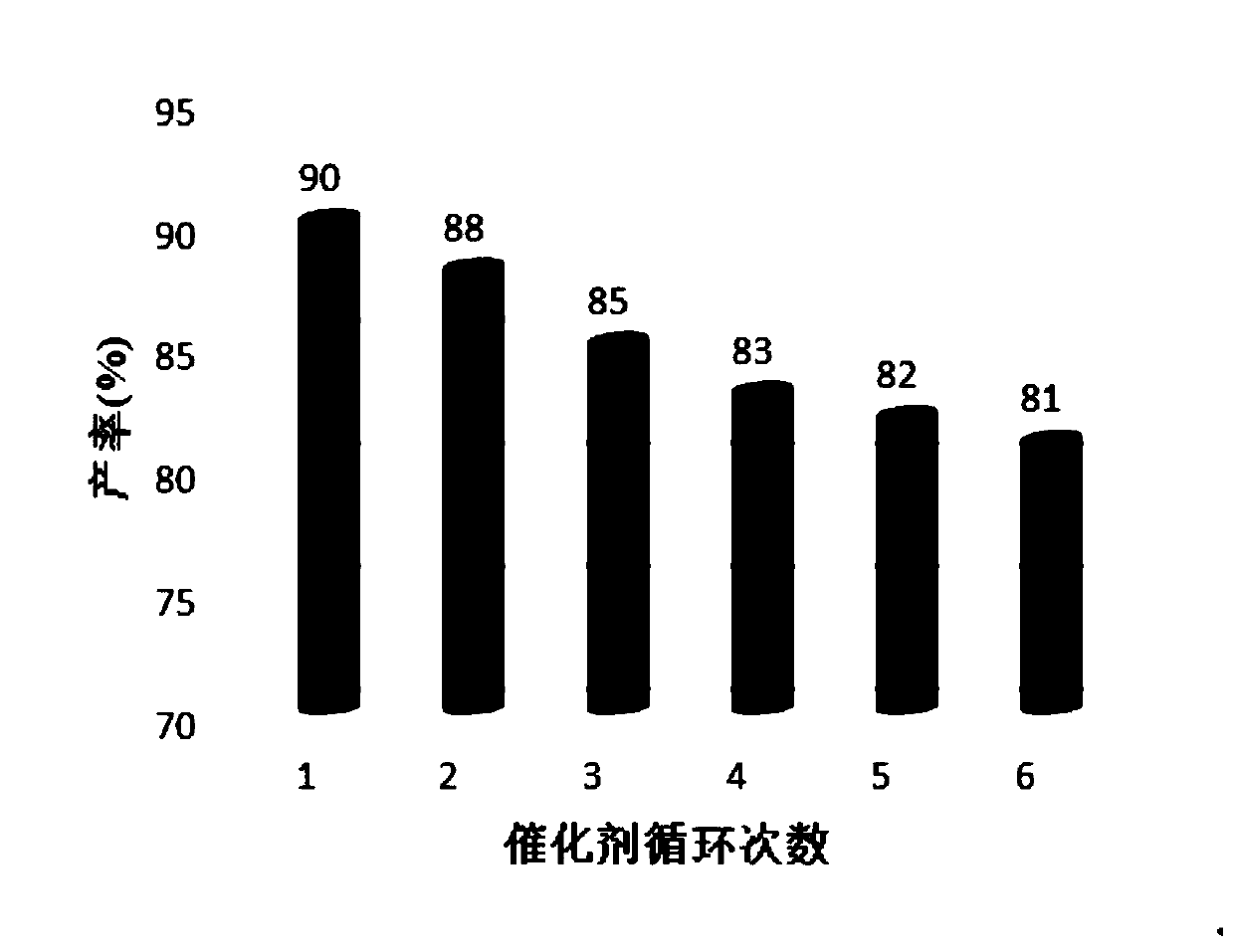
[0060] (3) The reactor was heated to 90° C. to start the reaction by microwave heating, cooled to room temperature after 1 hour of reaction, and ethyl acetate was added into the reactor, and stirred to dissolve. Suction filtration after standing still, and the filtrate can be further recrystallized through a mixed solvent of ethanol and water or purified by column chromatography after vacuum distillation. 2,3-Diphenylquinazolin-4(1H)-one was obtained in 88% yield. The filter cake is dried to become a heteropolyacid ionic liquid catalyst, which can be recycled and stored, and can also be recycled and reused at least 6 times.
[0061] 2,3-Diphenylquinazolin-4(1H)-one: 2,3-diphenylquinazolin-4(3H)-one
[0062] White soil. Mp: 148.1-150.9℃; 1H NMR (400MHz, CDCl3) δ8.36 (d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com