A liquid fluororubber composition based on coordination crosslinking, its preparation method and application
A liquid fluororubber, coordination cross-linking technology, applied in the field of fluorine materials, can solve the problems of slow reaction rate, long time, long curing time, etc., and achieve the effect of easy operation, simple reaction process and short curing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A method for preparing a liquid fluororubber composition based on coordination crosslinking, comprising the steps of:
[0058] In a 100ml round bottom flask, add 10ml acetone, add 10g carboxyl-terminated liquid fluororubber (vinylidene fluoride-hexafluoropropylene copolymer, carboxyl content is 2.98wt%, number average molecular weight is 3689) under mechanical stirring, stir at room temperature for 30min , until the carboxyl-terminated liquid fluororubber is completely dissolved;
[0059] Dissolve 0.73g of anhydrous zinc acetate in 7.3g of methanol / water (v:v=1:1) solution to prepare methanol / water solution of anhydrous zinc acetate; then add it to carboxyl-terminated liquid fluororubber and stir at room temperature After 1h, dry at 60°C to remove the solvent to obtain intermediate product A;
[0060] Add intermediate product A to 30ml DMF, stir at room temperature until it is completely dissolved, add 0.76g poly(4-vinylpyridine-co-styrene) (PSVP), stir at room tempera...
Embodiment 2-5
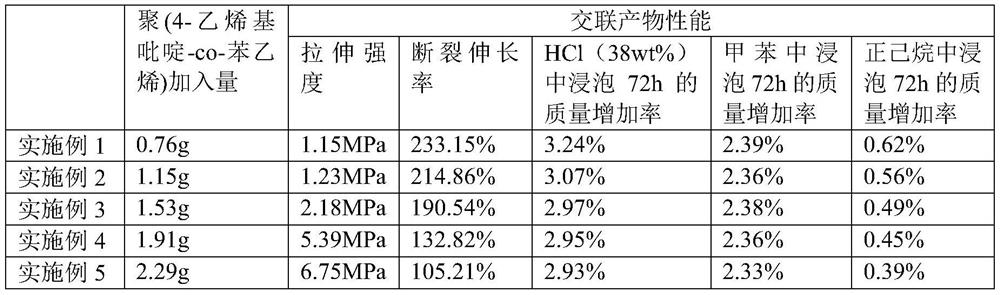
[0064] The preparation methods of the liquid fluororubber compositions based on coordination crosslinking provided by Examples 2-5 are the same as those in Example 1, the only difference being that the amount of poly(4-vinylpyridine-co-styrene) is different, see for details Table 1.
[0065] Wherein, the molar ratio of the carboxyl group of the carboxyl-terminated liquid fluororubber in Example 2, the metal ion in the metal salt, and the pyridine group in the pyridyl-containing polymer is 1:0.6:1.5;
Embodiment 3
[0066] In Example 3, the carboxyl group of the carboxyl-terminated liquid fluororubber, the metal ion in the metal salt, and the pyridine group in the pyridyl-containing polymer have a molar ratio of 1:0.6:2;
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com