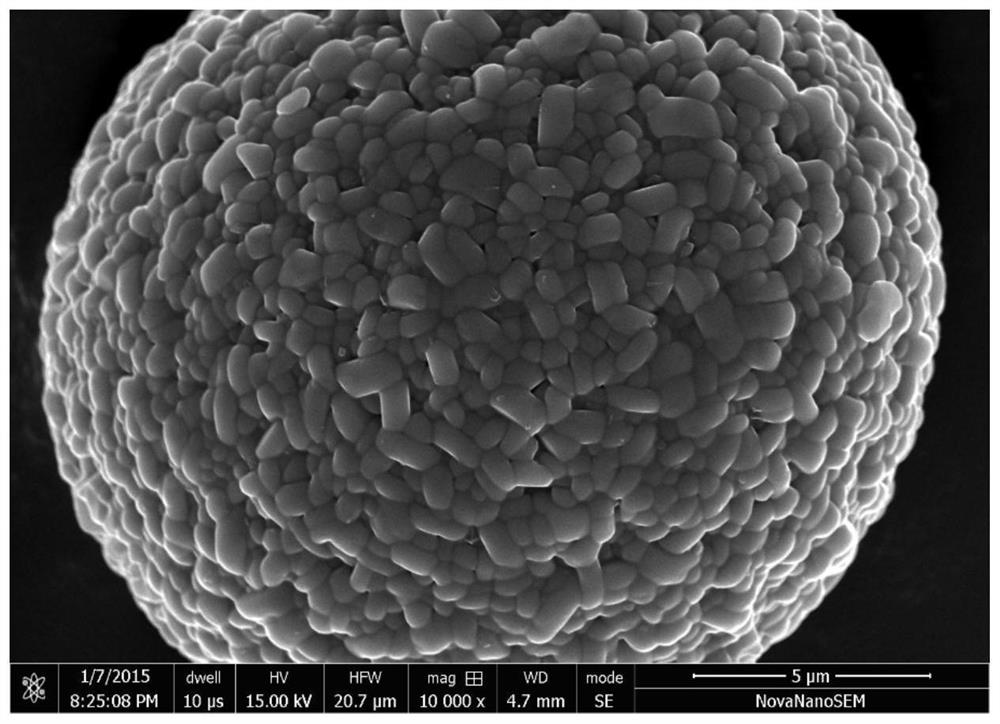
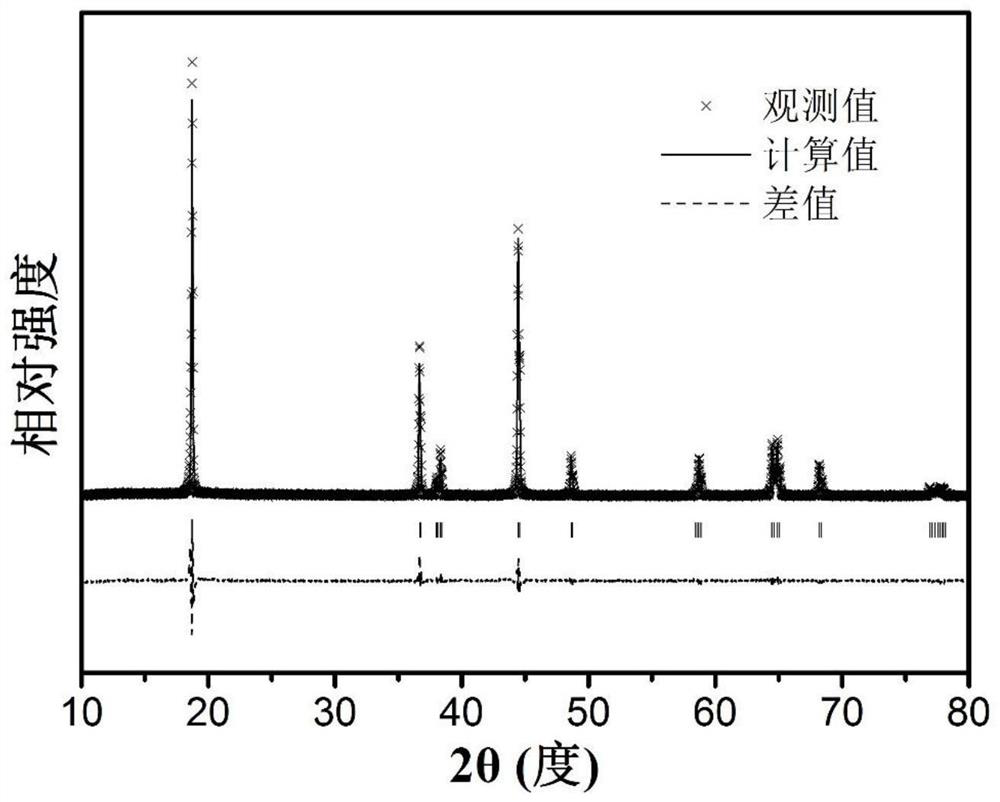
Sintering process of a high-stability lithium-ion high-nickel cathode material
A cathode material, high-stability technology, applied in nickel compounds, structural parts, electrode manufacturing, etc., can solve the problems of high degree of lithium-nickel mixing, increasing the difficulty of material storage and processing, and lithium-nickel mixing, etc. The effect of lithium-nickel mixing degree, shortening sintering time, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
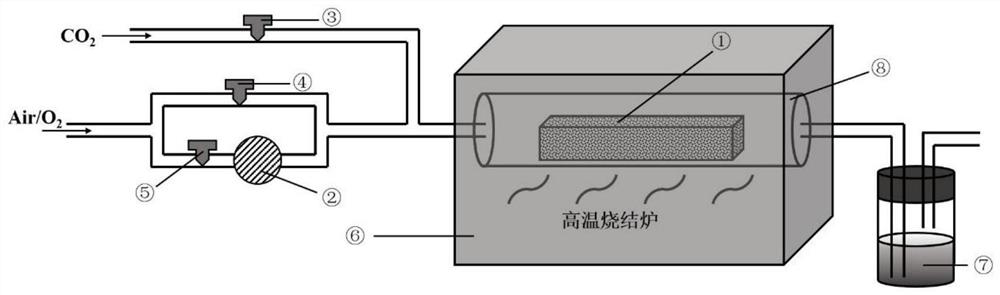
[0028] In this embodiment, the sintering process for preparing a high-nickel positive electrode material for a high-stability lithium-ion battery is based on a high-temperature sintering furnace 6, and the high-temperature sintering furnace 6 is provided with a corundum tube 8 with a porcelain boat 1 inside. The gas port is provided with a carbon dioxide channel with a valve, an oxygen channel and an ozone channel in parallel, an ozone generator 2 is also provided on the ozone channel, and an exhaust gas absorption device 7 is provided at the gas outlet of the corundum tube 8,
[0029] Described sintering process comprises the steps:
[0030] S1, the high-nickel cathode material precursor Ni 0.8 co 0.1 mn 0.1 (OH) 2 and lithium salt LiOH·H 2 O is mixed evenly according to the molar ratio of 1:1.05 according to the molar ratio, and the mixture after mixing is placed in porcelain boat 1, then porcelain boat 1 is put into high-temperature sintering furnace 6, seals, checks th...
Embodiment 2~5
[0038] On the basis of Example 1, change the number of times that the ozone gas is passed into, and the times of the ozone gas into the embodiments 2 to 5 are respectively 1 time, 2 times, 4 times, 5 times, and do not change the time of passing into the ozone gas each time , That is to say, the ozone gas was passed through for 20 min, 40 min, 80 min, and 100 min in total in Examples 2 to 5. The cycle performance of button batteries assembled with high-nickel cathode materials obtained under different conditions is shown in Table 1 (the charge and discharge current density is 100mA / g), and the influence of the time of ozone introduction on the battery cycle performance was discussed.
[0039] Table 1: The influence of the time of passing ozone on the cycle performance of the battery
[0040]
[0041] It can be seen from Examples 2 to 5 that, under the condition that other conditions remain unchanged, the longer the ozone time is, the higher the capacity retention rate of the...
Embodiment 6~9
[0043]On the basis of Example 1, the highest sintering temperature was changed, and the highest sintering temperatures of Examples 6-9 were 720°C, 740°C, 780°C, and 800°C, respectively. The cycle performance of the button battery assembled with the high-nickel cathode material obtained under different conditions is shown in Table 2 (the charge and discharge current density is 100mA / g), and the influence of the highest sintering temperature on the cycle stability of the battery is discussed.
[0044] Table 2: Effect of maximum sintering temperature on battery cycle stability
[0045]
[0046] It can be seen from Examples 6-9 that the maximum sintering temperature has a great influence on the cycle performance of the material, and the cycle performance of the material will be deteriorated if the sintering temperature is too low or too high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com