Preparation method of sulfonic acid type aromatic block cation exchange resin
A cation exchange and sulfonic acid type technology, applied in the direction of cation exchange materials, can solve the problems of ion exchange resins that have not been reported, and achieve the effect of increasing the quantity, increasing the free volume, and improving the adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
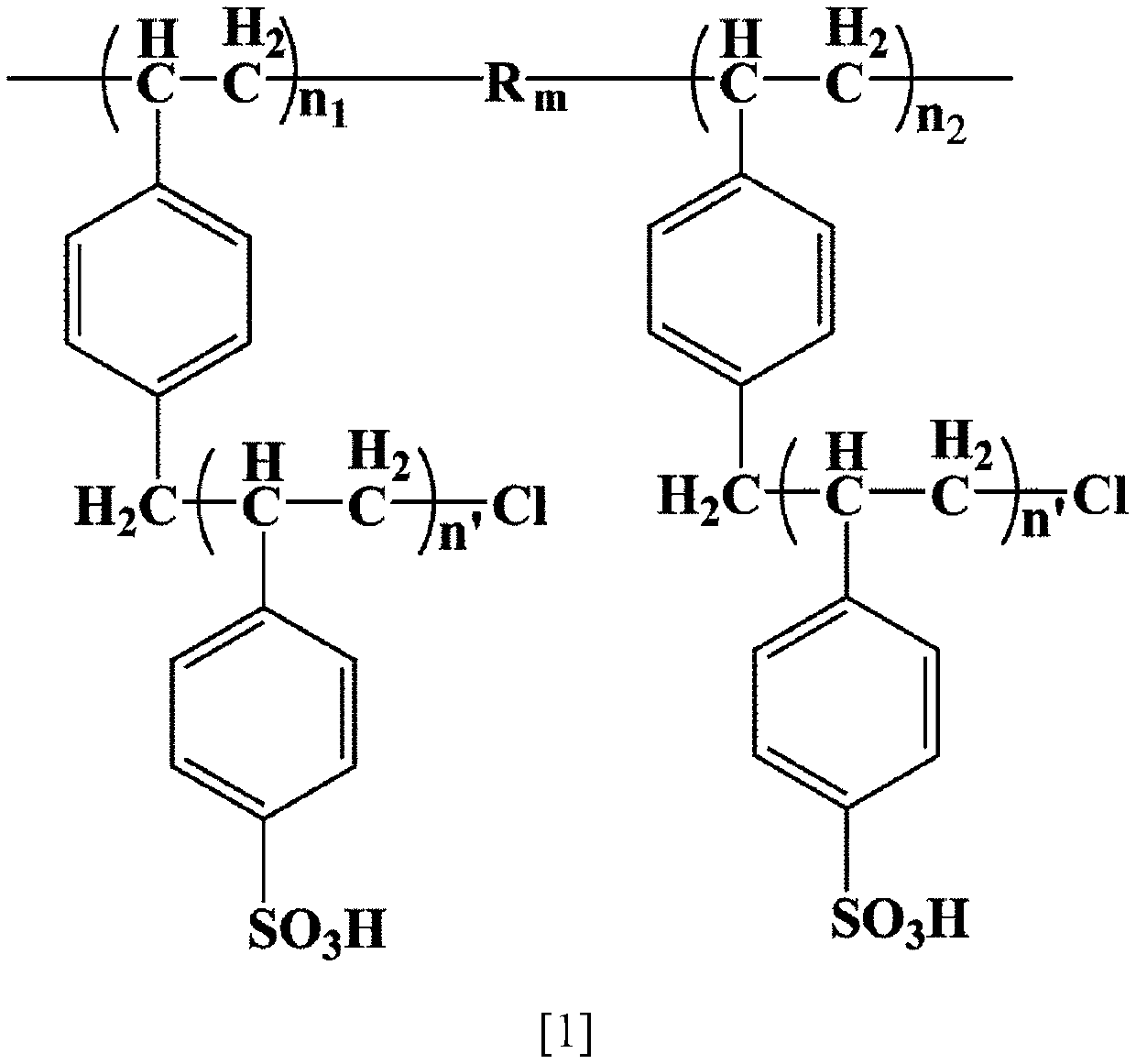
[0026] A kind of preparation method of sulfonic acid type aromatic block type cation exchange resin of the present invention comprises the following steps:
[0027] a. Dissolve the block polymer in chloroform to obtain a polymer solution with a mass fraction of 0.5 to 20 wt%, place the polymer solution in an ice-water bath (<2°C), and then add the mass fraction to the polymer solution 1~25% chloromethylation reagent trimethylchlorosilane or 1,4-dichloromethoxybutane and 0.25~6.25% catalyst anhydrous tin tetrachloride, react in the above ice-water bath for 10~60min After that, move to normal temperature to react for 6-10 hours, then move to normal temperature to react. After the reaction, drop the reaction mixture into the precipitant propanol, isobutanol or acetone to obtain a precipitate; filter the precipitate with a suction filter funnel, Wash the obtained precipitate with 200-400 mL of propanol, and then dry the washed precipitate in an oven at 25-80°C for 12-24 hours to o...
Embodiment 1
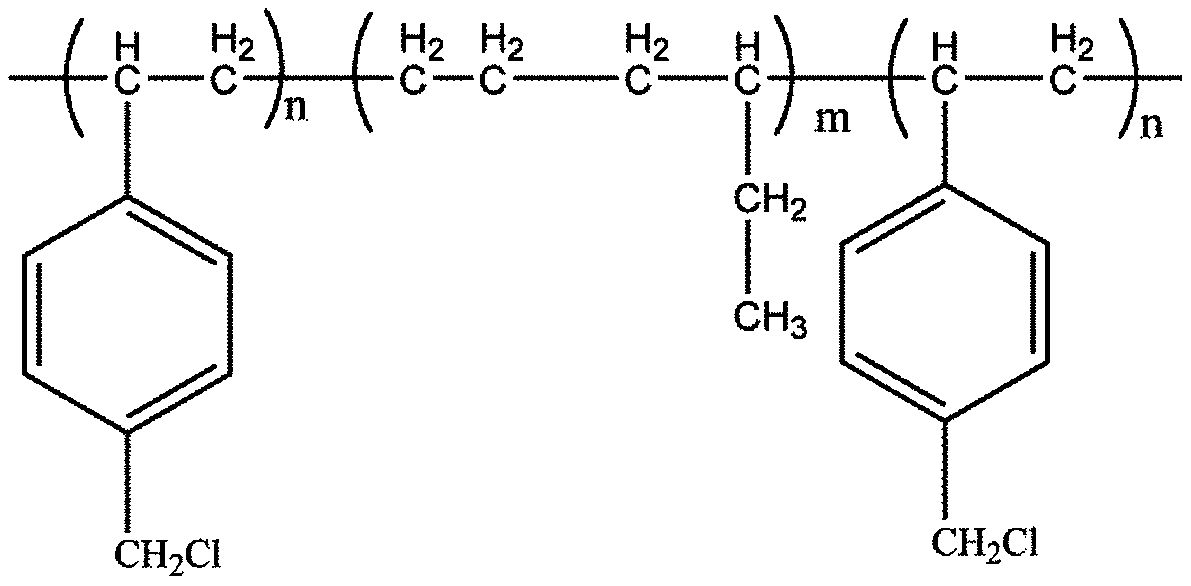
[0033] The block polymer used is polystyrene-ethylene / butylene-styrene (SEBS), where R is a combination of one molecule of ethylene and one molecule of 1-butene monomer, m=400, n 1 =n 2 =n=30, n'=6.
[0034] (1) Chloromethylation reaction: 10.0g SEBS was dissolved in 250mL chloroform to obtain a polymer solution with a mass fraction of 2.6wt%, and the polymer solution was sequentially added in an ice-water bath (0°C) with a mass fraction of 9.5 % of trimethylchlorosilane and 3.2% anhydrous tin tetrachloride, reacted for 30 minutes under the above ice-water bath conditions, continued the reaction for 6 hours at room temperature, added a mass fraction of 120% propanol solution to precipitate the polymer, and baked it in an oven at 25°C Dry in medium for 24h to obtain the chloromethylation product SEBS@CH with a chloromethylation rate of 43% 2 Cl, whose structure is shown below:
[0035]
[0036] (2) Grafting polystyrene reaction: 2g chloromethylated block polymer is mixed wi...
Embodiment 2
[0041] Using the raw material SEBS in Example 1, the SEBS-g-PSt polymer solution was prepared according to the chloromethylation reaction and grafted polystyrene reaction in Example 1. Different from Example 1, in the obtained SEBS-g-PSt polymer solution, a mass fraction of 200% styrene was added as a porogen, and then the mixed solution was added dropwise to 400mL propanol solution to precipitate polymer resin The particles were finally dried in an oven at 50° C. for 24 hours to obtain polystyrene-grafted SEBS (SEBS-g-PSt-2) spherical particles after adding porogen. Then carry out sulfonation according to the sulfonation method in Example 1 to obtain the sulfonic acid type cation exchange resin S-SEBS-g-PSt-2 with an ion exchange capacity of 2.18.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com