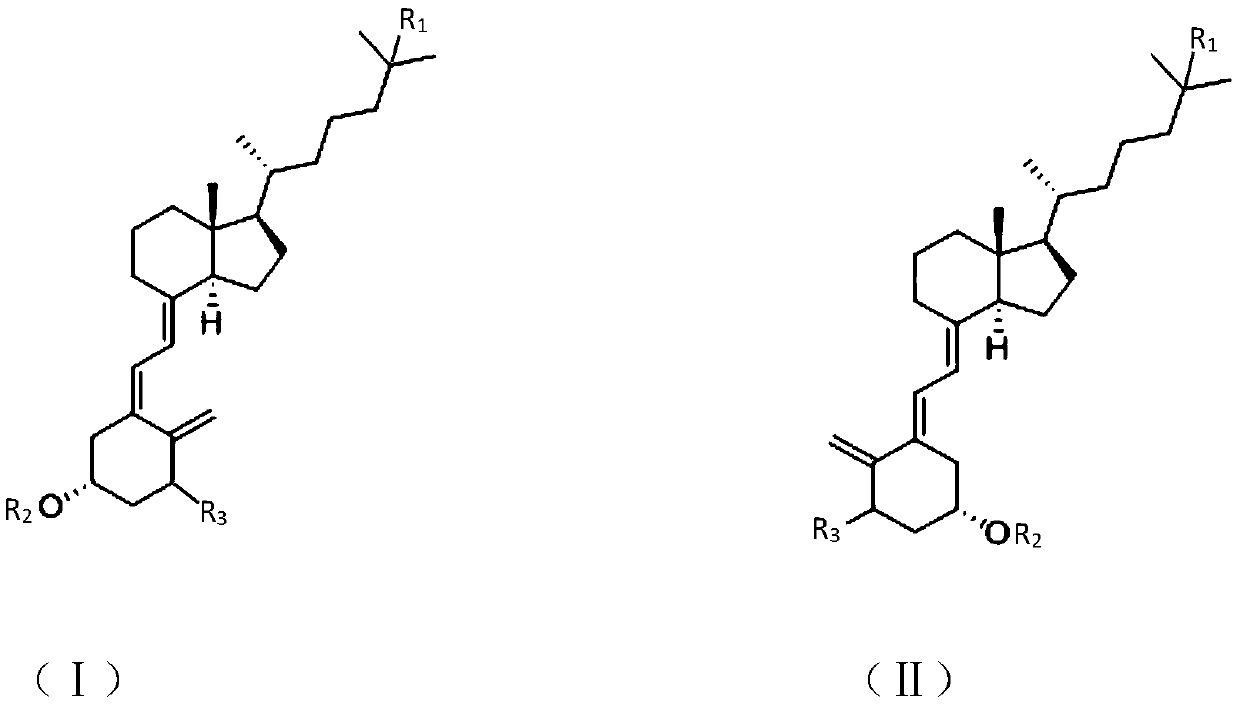
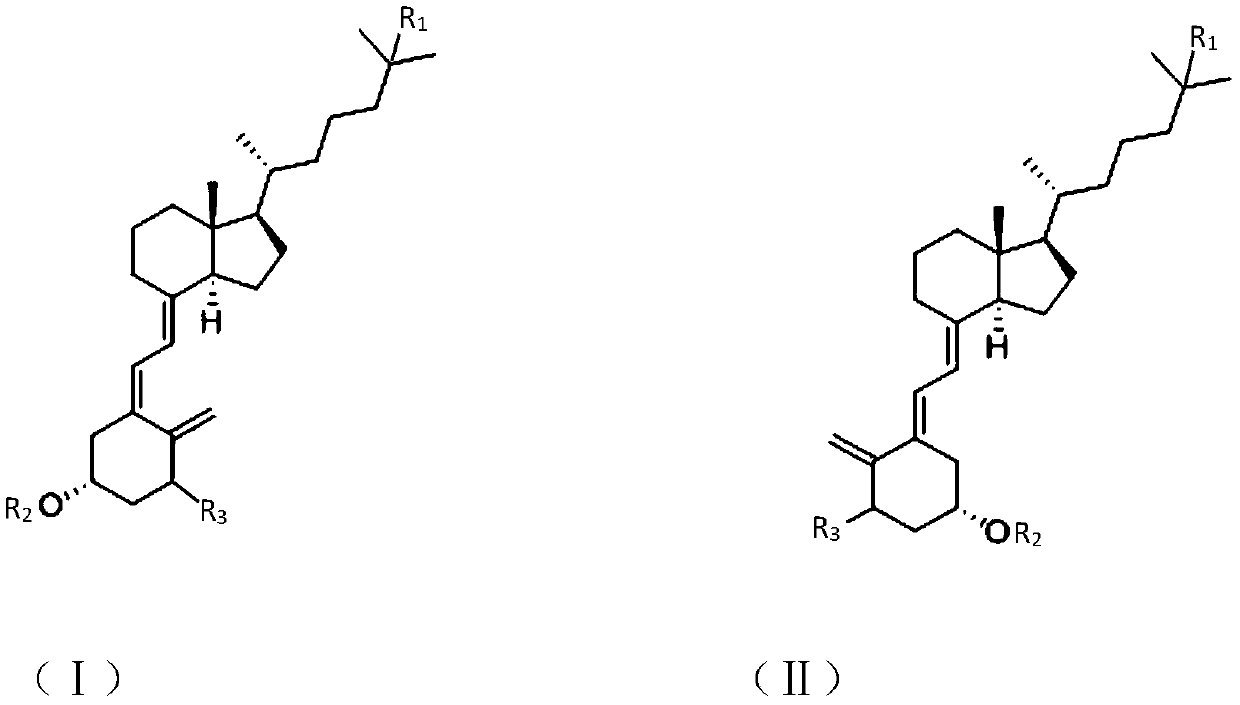
Synthesis method of vitamin D analogue intermediate
A synthetic method and analog technology, applied in the field of vitamin D analog intermediates, can solve the problems of inability to directly hydroxylate, low yield, complicated operation, etc., and achieve the effect of shortening the production cycle and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
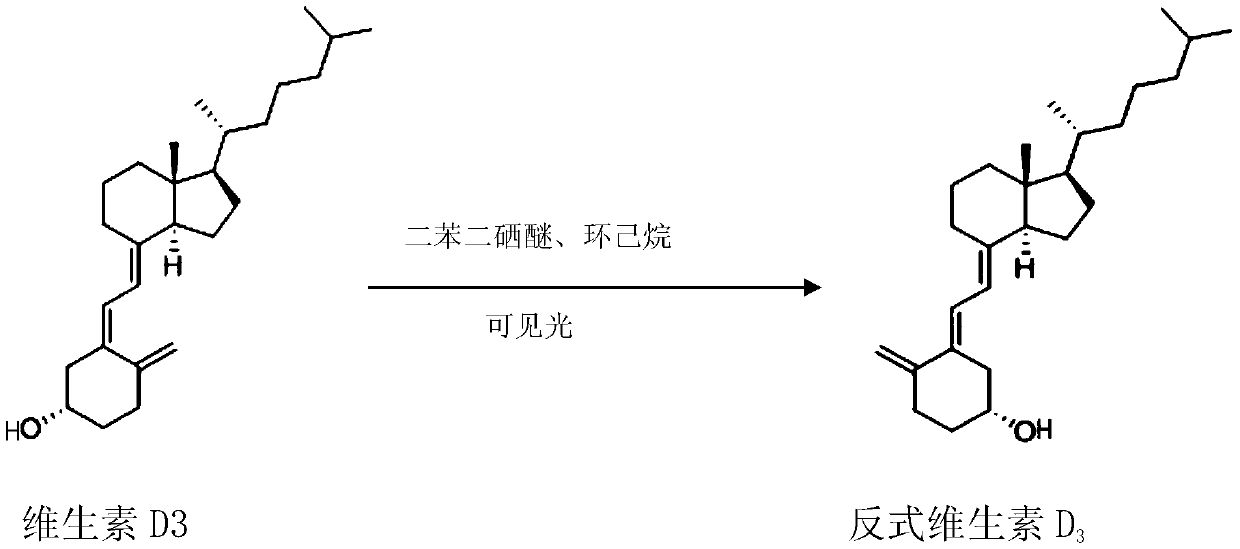
[0021] Example 1 trans vitamin D 3 preparation of
[0022]
[0023] 20 g vitamin D at room temperature 3 and 0.156g of diphenyldiselenide were added to a 1000ml flask, and then 500ml of cyclohexane was added to dissolve it. Irradiate with a 250-watt incandescent lamp, collect samples at 30 minutes, 60 minutes, 90 minutes and 120 minutes respectively, and analyze the reaction progress by HPLC. As shown in Table 1 below, the results indicated that the reaction was completed in 1.5 hours with a conversion rate of 66%.
[0024] Table 1 conversion rate table
[0025] time (min) Vitamin D 3 %
[0026] Subsequently, the reaction mixture was spun to dryness at 35°C, and pure trans-vitamin D was obtained by preparative liquid phase separation 3 , as an intermediate for the synthesis of alfacalcidol raw materials. unreacted vitamin D 3 After purification and separation, it can be recycled again.
Embodiment 2
[0027] The preparation of embodiment 2 trans-calcifediol
[0028]
[0029] Add 10g of calcifediol and 0.078g of diphenyldiselenide into a 1000ml flask at room temperature, then add 250ml of n-hexane and 250ml of ethyl acetate to dissolve. Irradiate with a 250-watt tungsten halogen lamp, collect samples at 30 minutes, 60 minutes, 90 minutes and 120 minutes respectively, and analyze the reaction progress by HPLC. As shown in Table 2 below, the results indicated that the reaction was completed in 1.5 hours with a conversion rate of 70%.
[0030] Table 2 conversion rate table
[0031] time (min) Calcidiol% Trans-calcifediol% 30 75 25 60 50 50 90 30 70 120 30 70
[0032] Subsequently, the reaction mixture was rotary evaporated to dryness at 35° C., and pure trans-calcifediol was obtained by preparative liquid phase separation, which was used as an intermediate for the synthesis of calcitriol raw materials. The unreacted calcifediol can...
Embodiment 3
[0033] Example 3 Preparation of Intermediate (IV).
[0034]
[0035] 10g vitamin D 3 , 0.55g of dibutylhydroxytoluene and 4.75g of imidazole were placed in the reaction flask and dissolved in 150ml of dichloromethane, and 5g of tert-butyldimethylsilyl chloride was dissolved in 25ml of dichloromethane, and slowly added dropwise to the above In the reaction bottle, stirring at room temperature, HPLC detection of vitamin D 3 The reaction is complete and intermediate (Ⅲ) is generated. After further purification by preparative liquid phase, pure intermediate (Ⅲ) was obtained
[0036] At room temperature, add 10 g of intermediate (Ⅲ), 0.085 g of dibenzyl diselenide and 0.054 g of diethyl diselenide into a 1000 ml flask, and then add 250 ml of cyclohexane and 250 ml of ethanol to dissolve. Irradiate with a 250-watt halogen lamp, collect samples at 30 minutes, 60 minutes, 120 minutes and 180 minutes, respectively, and analyze the reaction progress by HPLC. As shown in Table 3 b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com