Method for fluorinating volatilizing recovery of uranium by using nitrogen trifluoride as fluorinating agent
A nitrogen trifluoride and uranium recovery technology, applied in chemical instruments and methods, plutonium halide, plutonium compounds, etc., can solve the problems of process equipment corrosion, poor safety, short service life, etc., and achieve low chemical toxicity and high safety. , the effect of long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Recovery of uranium in molten salt system of uranium-containing compound
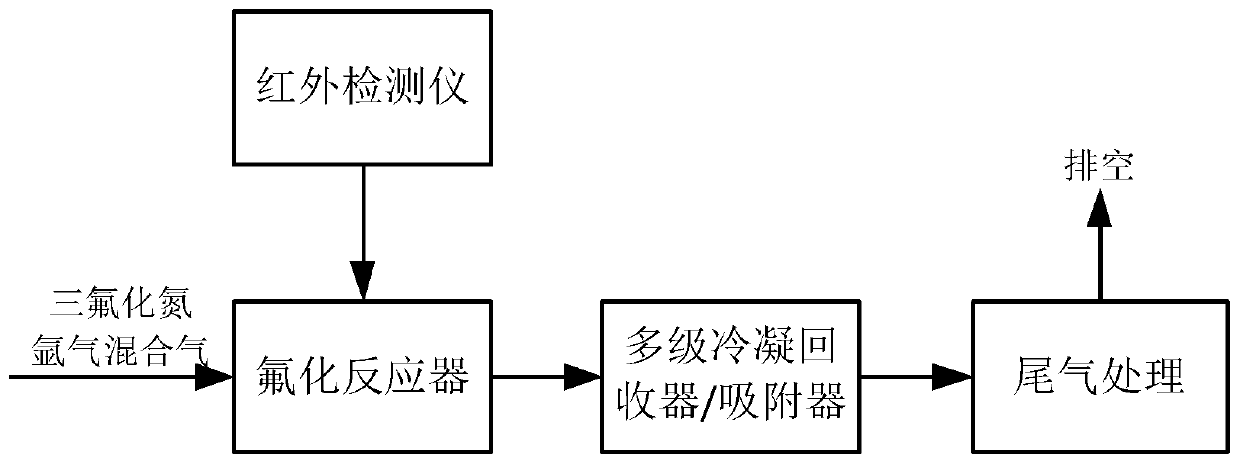
[0061] (1) Will contain 3gUF 4 KF-ZrF 4 A total of 100g is placed in the reactor, the reactor lid is covered, and the atmosphere in the reactor is replaced with pure argon.
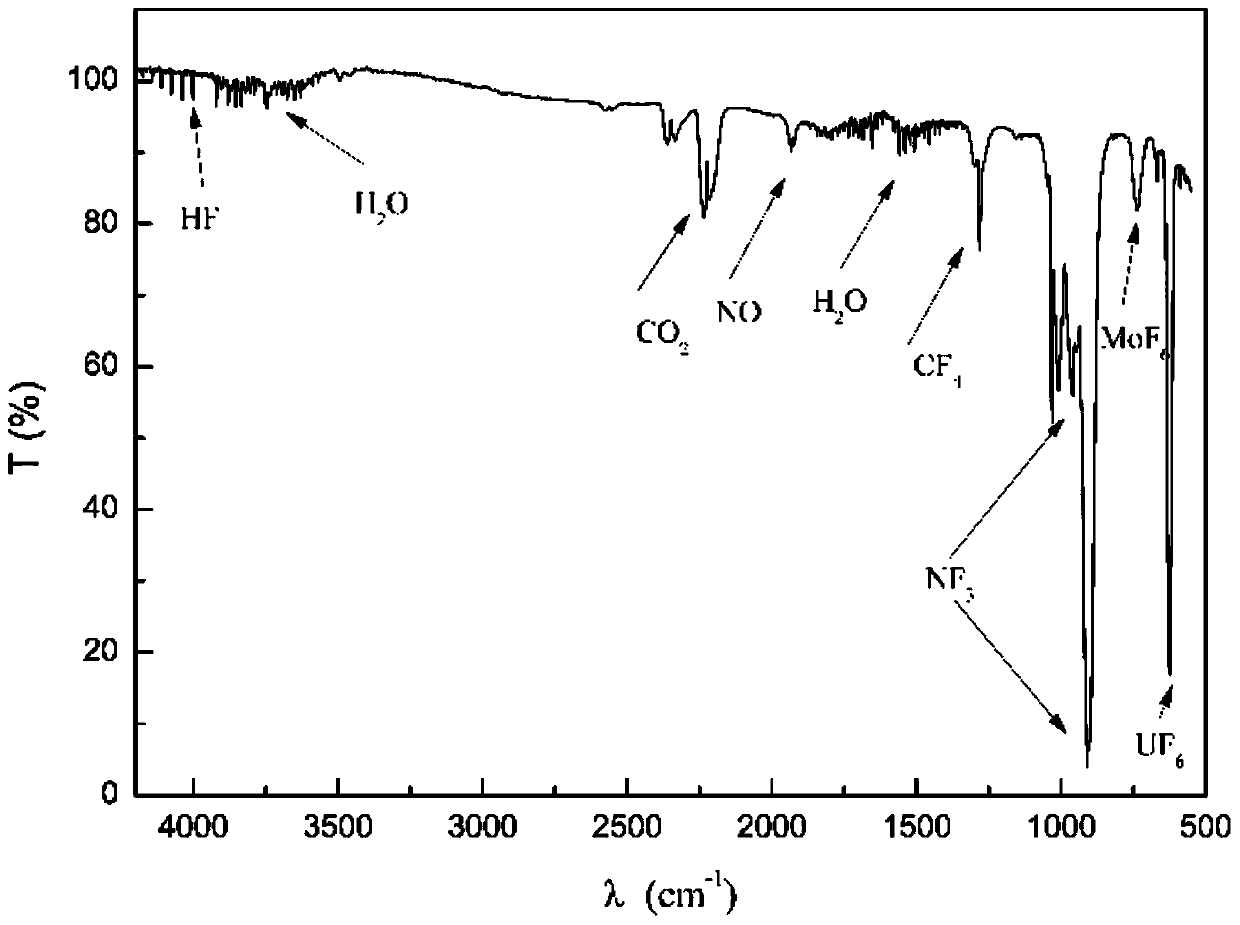
[0062] (2) Heat the reactor to increase the temperature from room temperature to 200°C, and then keep it for 2 hours; at the end of the temperature keeping, replace the atmosphere in the reactor with pure argon. The gas pipelines are heated to 100°C; a two-stage condenser is used, and the condenser temperature is -60°C. Continue to heat the reactor until the temperature reaches 700°C; keep it for 3h until the molten salt is completely melted, and pass a mixed gas of nitrogen trifluoride and argon (NF) into the molten fluoride salt in the reactor through a mass flow meter 3 The volume fraction is 50%), the flow rate is 0.2L / min, and the outlet gas of the reactor is detected by a Fourier infrared spectrometer.
[0063] (3)...
Embodiment 2 4
[0066] Example 2 Recovery of uranium in uranium tetrafluoride
[0067] (1) Put 50g UF 4 Place in the reactor, cover the reactor cover, and replace the atmosphere in the reactor with pure argon.
[0068] (2) Heat the reactor to increase the temperature from room temperature to 250°C, then keep it for 3 hours; at the end of the temperature keeping, replace the atmosphere in the reactor with pure argon. The gas pipelines are all heated to 100°C; the product is recovered by a two-stage adsorber with sodium fluoride as the adsorbent, and the temperature of the adsorber is 100°C. Continue to heat the reactor until the temperature reaches 500℃; keep it for 2h, and pass the mixed gas of nitrogen trifluoride and argon (NF 3 The volume fraction is 5%), the flow rate is 1L / min, and the outlet gas of the reactor is detected by a Fourier infrared spectrometer.
[0069] (3) UF in the reactor outlet gas after 2h 6 When the concentration is lower than the lower limit of infrared detection, stop fee...
Embodiment 3
[0072] Example 3 Recovery of uranium in molten salt system of uranium-containing compounds
[0073] (1) Will contain 20gUF 4 NaF-ZrF 4 A total of 1000 g is placed in the reactor, the reactor lid is covered, and the atmosphere in the reactor is replaced with pure argon.
[0074] (2) Heat the reactor to increase the temperature from room temperature to 250℃, then keep it for 3h; at the end of the heat preservation, replace the atmosphere in the reactor with pure argon; the gas pipelines are heated to 100℃; adopt two-stage condensation, the condenser temperature They are -40°C and -70°C respectively. Continue to heat the reactor until the temperature reaches 750°C; keep for 24 hours until the molten salt is completely melted, and pass a mixed gas of nitrogen trifluoride and argon (NF) into the molten fluoride salt in the reactor through a mass flow meter 3 The volume fraction is 10%), the flow rate is 2L / min, and Fourier infrared spectrometer is used to detect the outlet gas of the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com