A kind of synthetic method of semigossypol, gossypol and their analogs
A synthetic method, the technology of semi-gossypol, which is applied in the field of gossypol and their analogs, and the synthesis of semi-gossypol, can solve the problem of single product structure, difficult synthesis of semi-gossypol and gossypol analogs, and inability to obtain semi-gossypol and gossypol Gossypol analogs and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
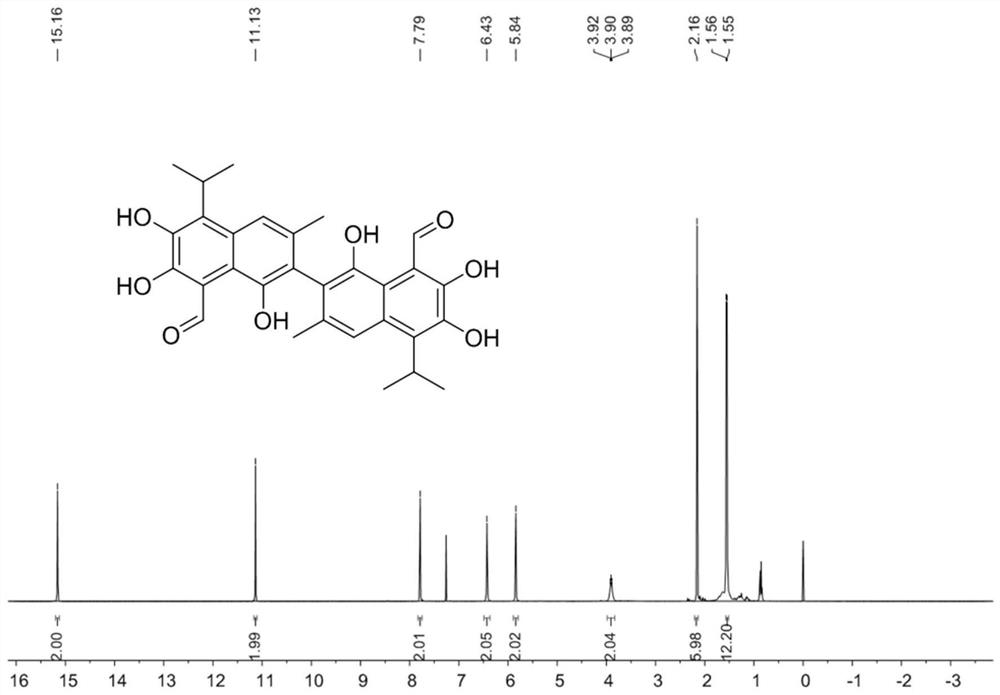
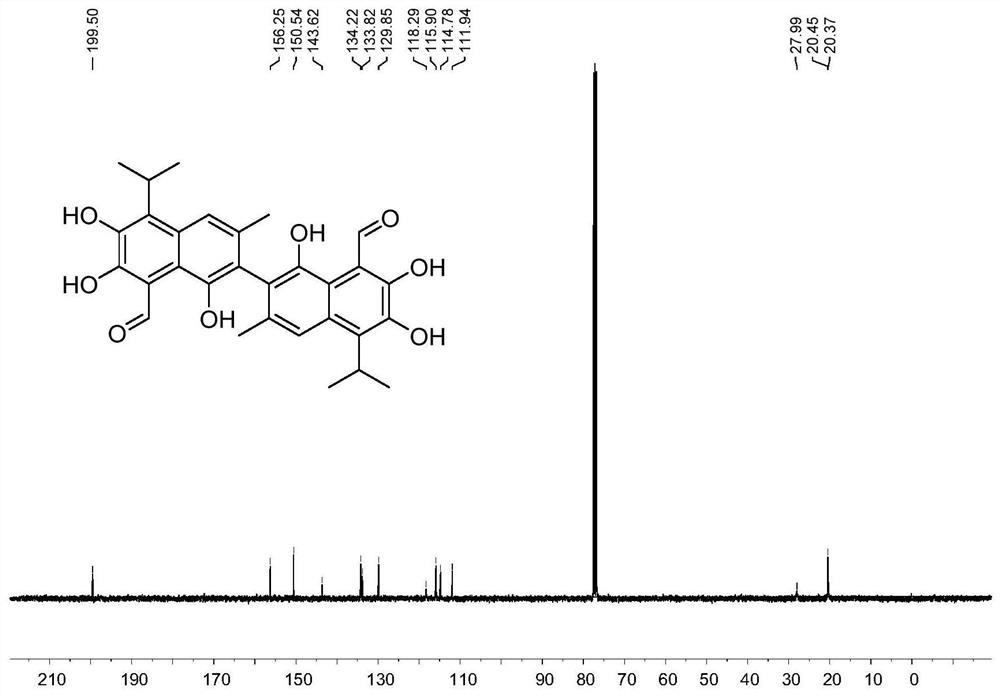
[0142] The synthesis of gossypol (compound X VI-1), synthetic route is as follows:
[0143]
[0144] Concrete synthetic steps are as follows:
[0145] (1) Add 6.8g of compound I-1 isopropyl maltol, 3 equivalents of potassium carbonate and 1.2 equivalents of propargyl bromide to 150 ml of acetonitrile, heat at 80°C for 14 hours, cool to room temperature, filter, After the solvent was evaporated under reduced pressure, 8.0 g of compound II-1 was obtained by column chromatography with a yield of 94%. 1 H NMR (400MHz, CDCl 3 )δ7.71(d, J=5.6Hz, 1H), 6.35(d, J=5.6Hz, 1H), 4.91(d, J=2.4Hz, 2H), 3.54(hept, J=7.0Hz, 1H) , 2.46(t, J=2.4Hz, 1H), 1.23(d, J=7.0Hz, 6H). 13 C NMR (101MHz, CDCl 3 )δ175.19, 167.55, 153.96, 141.01, 116.92, 79.21, 75.76, 58.72, 27.21, 19.92.HR-MS(ESI)calcd.for[C 11 h 12 o 3 +Na] + 215.0679, found: 215.0678.
[0146] (2) 8.0 g of compound II-1, 3 equivalents of potassium carbonate, 1.5 equivalents of allyl bromide, 0.1 equivalents of cuprous iodide an...
Embodiment 2
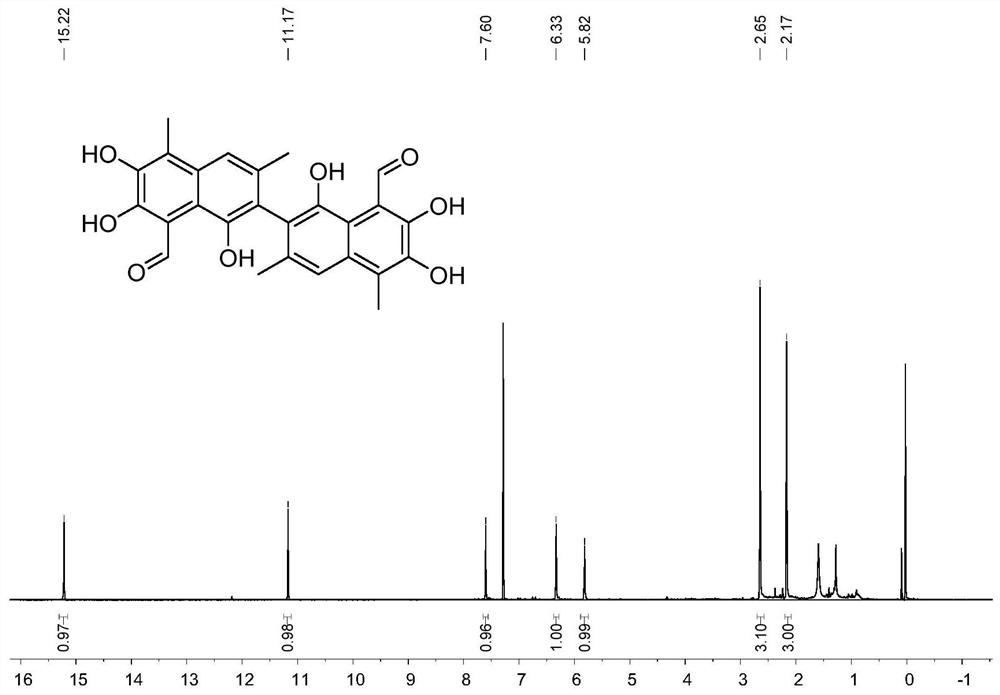
[0164] The synthesis of methyl-gossypol (compound XVI-2), the synthetic route is as follows:
[0165]
[0166] Concrete synthetic steps are as follows:
[0167] (1) Add 15g of compound I-2 methyl maltol, 3 equivalents of potassium carbonate and 1.2 equivalents of propargyl bromide to 300 ml of acetonitrile, heat at 80°C for 12 hours, cool down to room temperature, and use a short silica gel column After filtering and distilling off the solvent under reduced pressure, 17.2 g of compound II-2 was obtained by column chromatography with a yield of 88%. 1 H NMR (400MHz, CDCl3) δ7.63(d, J=5.6Hz, 1H), 6.39-6.30(m, 1H), 4.88(d, J=2.4Hz, 2H), 2.44(t, J=2.4Hz , 1H), 2.38(s, 3H). 13 C NMR (101MHz, CDCl3) δ174.84, 160.82, 153.77, 143.02, 117.29, 79.19, 75.75, 58.85, 15.42. HR-MS (ESI) calcd.for[C 9 h 8 o 3 +Na] + 187.0366, found 187.0367.
[0168] (2) 17.2 g of compound II-2, 3 equivalents of potassium carbonate, 1.5 equivalents of allyl bromide, 0.1 equivalents of cuprous iodid...
Embodiment 3
[0186] In Example 1, in step (1), 1.0 equivalent of potassium carbonate was added, 1.0 equivalent of propargyl bromide was added, and the reaction was carried out at 40° C., and the rest of the reaction conditions were unchanged. The yield of compound II-1 was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com