Preparation method of natural biflavone i3, ii8-biapigenin and ridiculuflavone A
A biflavone, natural technology, applied in the preparation method of new compounds and the field of medicine, can solve the problems of limited activity research, scarce sources, etc., and achieve the effect of wide biological activity and simple purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] In order to understand the present invention, the present invention will be further described below in conjunction with embodiment; Following embodiment is illustrative, not limiting, can not limit protection scope of the present invention with following embodiment.
[0020] The present invention provides compounds 1 and 2
[0021]
[0022] The present invention specifically includes compounds:
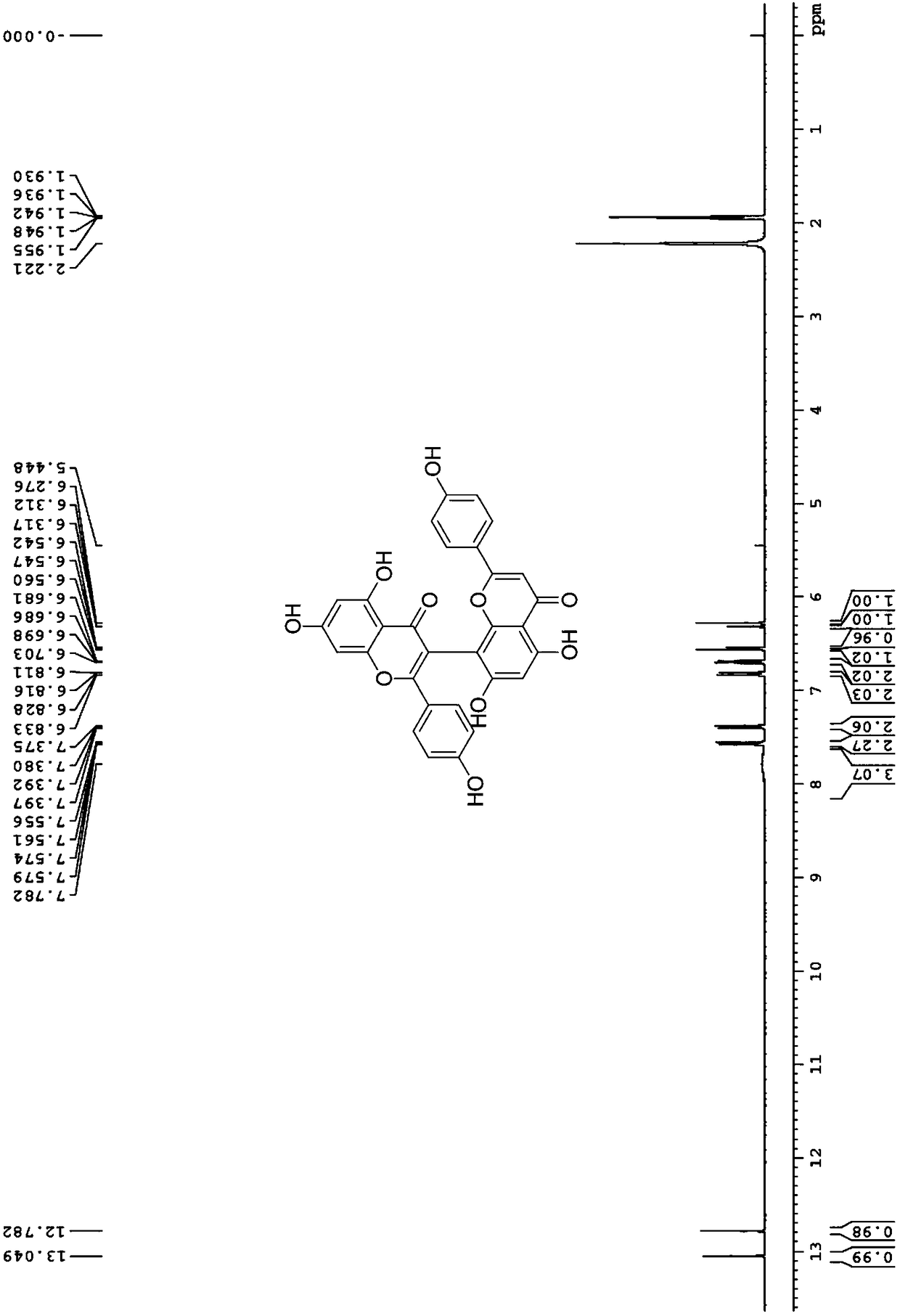
[0023] (1) 5,5',7,7'-tetrahydroxy-2,2'-bis(4-hydroxyphenyl)-4H,4'H-[3,8'-dibenzopyran]-4 ,4'-Diketone
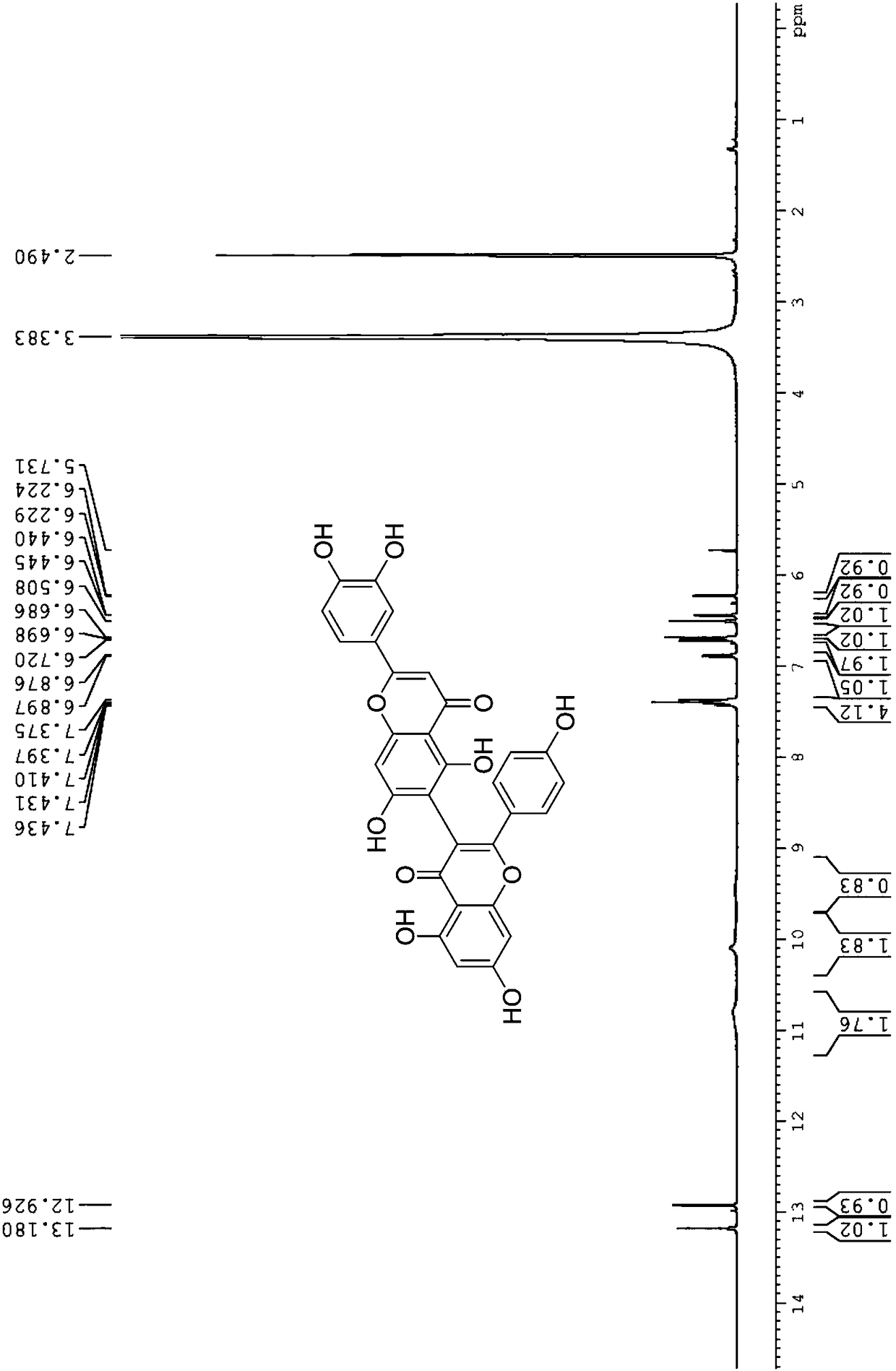
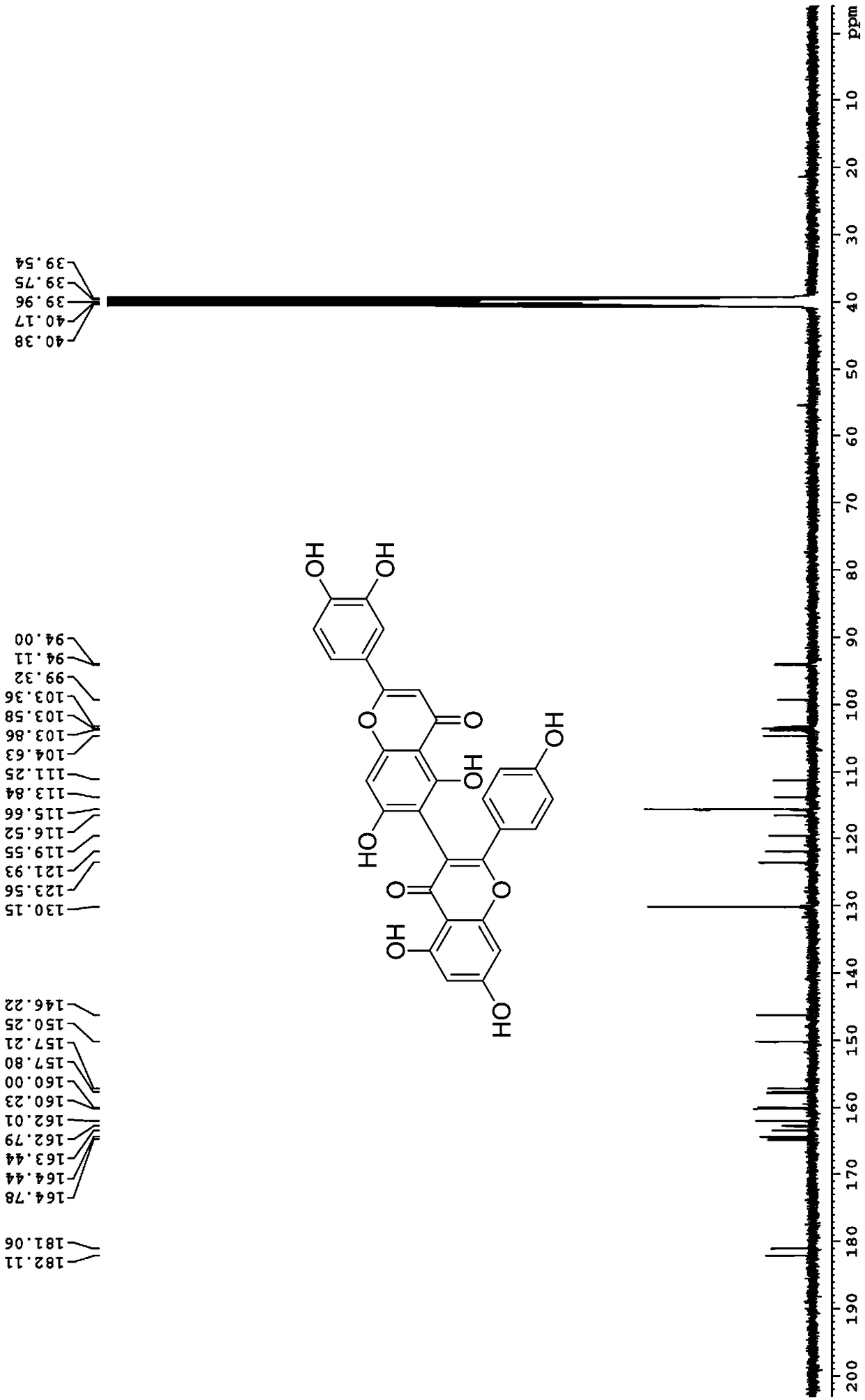
[0024] (2) 2'-(3,4-dihydroxyphenyl)-5,5',7,7'-tetrahydroxy-2-(4-hydroxyphenyl)-4H,4'H-[3,6 '-Dibenzopyran]-4,4'-dione
[0025] Synthetic routes of compounds 1 and 2
[0026]
[0027] Description 1
[0028] 5,5',7,7'-tetrahydroxy-2,2'-bis(4-hydroxyphenyl)-4H,4'H-[3,8'-dibenzopyran]-4,4' - dione
[0029] Add 2.0 g (0.010 mol) of 2-hydroxy-4,6-dimethoxyacetophenone and 2.0 g (0.012 mol) of 4-isopropoxybenzaldehyde into 3 mL of ethanol and stir for 5 min. Then 6ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com