Application of a kind of glycosyltransferase and its mutant in the synthesis of ginsenoside rh2
A technology of glycosyltransferase and ginsenoside, which is applied in the field of biopharmaceuticals to achieve the effects of simplified production process, low consumption and high output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Screening of glycosyltransferases for the synthesis of ginsenoside Rh2 by transglycosylation of protopanaxadiol
[0048] With the help of Rxnfinder, a search tool based on compounds and chemical reactions, combined with PIR, NCBI and other database resources, based on the principles of substrate similarity and catalytic reaction type, some glycosyltransferase genes that may catalyze the glycosylation of protopanaxadiol were screened. They are UGT51 from Saccharomyces cerevisiae, OleD from Streptomyces resistant and SGT2 from potato. Among them, the UGT51 clone derived from Saccharomyces cerevisiae was obtained, the OleD derived from Streptomyces resistant bacteria and the SGT2 derived from potato were obtained by total gene synthesis. The full-length ORFs of OleD and SGT2 genes were cloned into pET28a(+) plasmid with NdeI and XhoI restriction sites.
[0049] The genome of Saccharomyces cerevisiae S288c was extracted using a fungal genome extraction kit, and P...
Embodiment 2
[0061] Example 2 Separation, purification and structural identification of glycosylation products
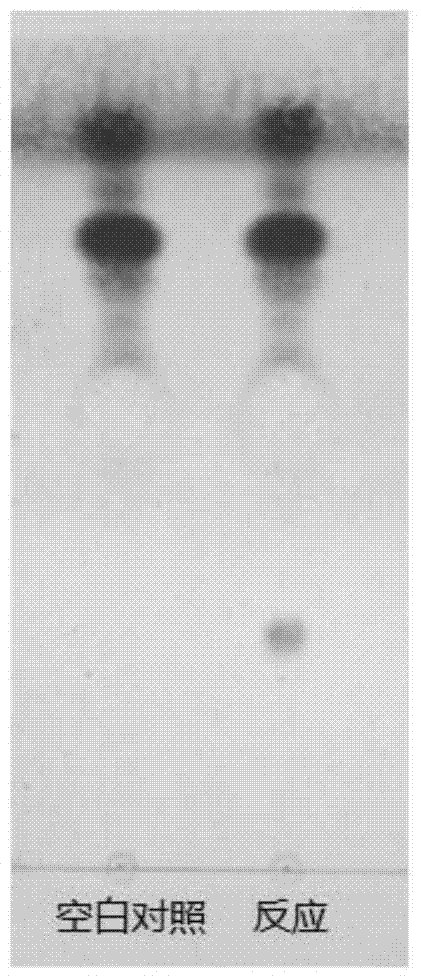
[0062]Prepare 100 mL of reaction solution according to the ratio in Example 1. Under the condition of 30°C, react for 48 hours. Add the same volume of n-butanol to terminate the reaction. Get the upper organic phase to dry by rotary evaporation, purify with silica gel column, eluent is chloroform:methanol=85:15, every 5mL aliquots are collected, the collected sample is carried out TLC analysis (condition is as described above), obtains protopanaxadiol Glycosylation product fraction, purity <90%.
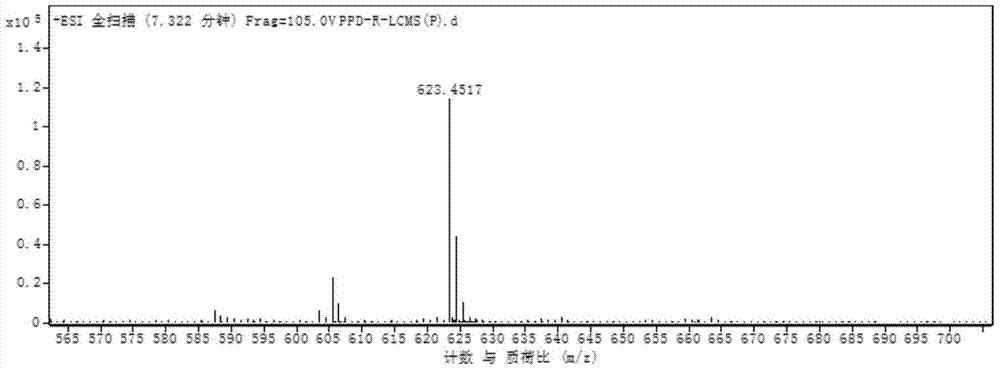
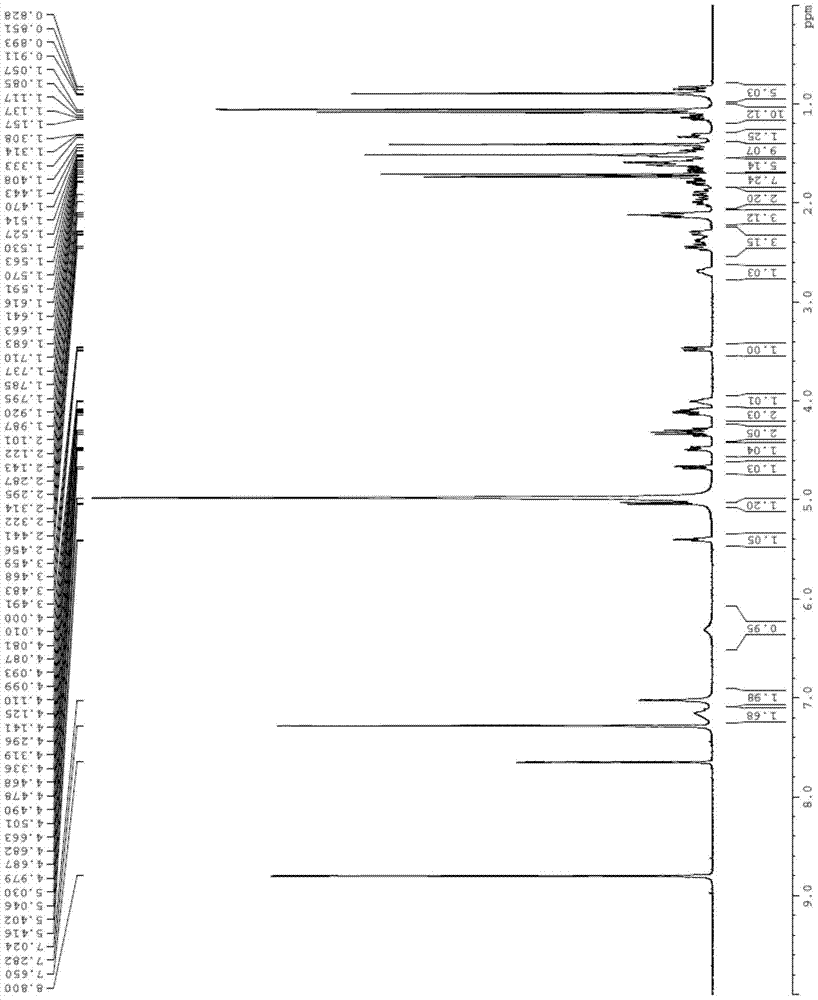
[0063] Further utilize Sep-Pak tC18 column (Waters) to carry out purification to above-mentioned collection part, water (A) and acetonitrile (B) are as eluent, adopt gradient elution (20%B, 40%B, 50%B, 60% B, 65% B, 70% B, 75% B, 80% B, 85% B, 90% B, 100% B), when eluted with 70% and 75% acetonitrile, protopanaxadiol glycosylation was obtained The product part has a purity of 98%. ...
Embodiment 3
[0064] Example 3 UGT glycosyltransferase wild-type in vitro enzymatic transformation to synthesize ginsenoside Rh2
[0065] Option One
[0066] According to Example 1, the crude enzyme solution of UGT51 glycosyltransferase was obtained.
[0067] Prepare the reaction solution with dimethyl sulfoxide (DMSO), protopanaxadiol, uridine diphosphate glucose (UDPG), Tris-HCl buffer, the proportion of organic solvent DMSO in the reaction solution is 10% (v / v), the original Panaxadiol is 0.5g / L, the molar concentration of Tris-HCl buffer is 50mmol / L, the pH is 7.5, and the ratio of UDPG to protopanaxadiol is 10:1 (w / w). Add UGT51 glycosyltransferase crude enzyme solution to the reaction solution at a ratio of 70% (v / v), react at 30° C., 180 rpm, for 48 hours.
[0068] Add the same volume of n-butanol to terminate the reaction, add methanol to dissolve the extracted organic phase after vacuum drying, and carry out HPLC detection analysis and quantification, the results are as follows ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com