Preparation method for tricyclic core skeleton of cycloheptatrienone natural product
A technology for cycloheptatrienone and natural products is applied in the field of preparation of the tricyclic core skeleton of cycloheptatrienone natural products, which can solve the problems of expensive reagents, cumbersome operations, long synthesis steps and the like, and achieves short and efficient strategies, high efficiency, high efficiency, and high efficiency. Efficient construction, overcoming the effects of long synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
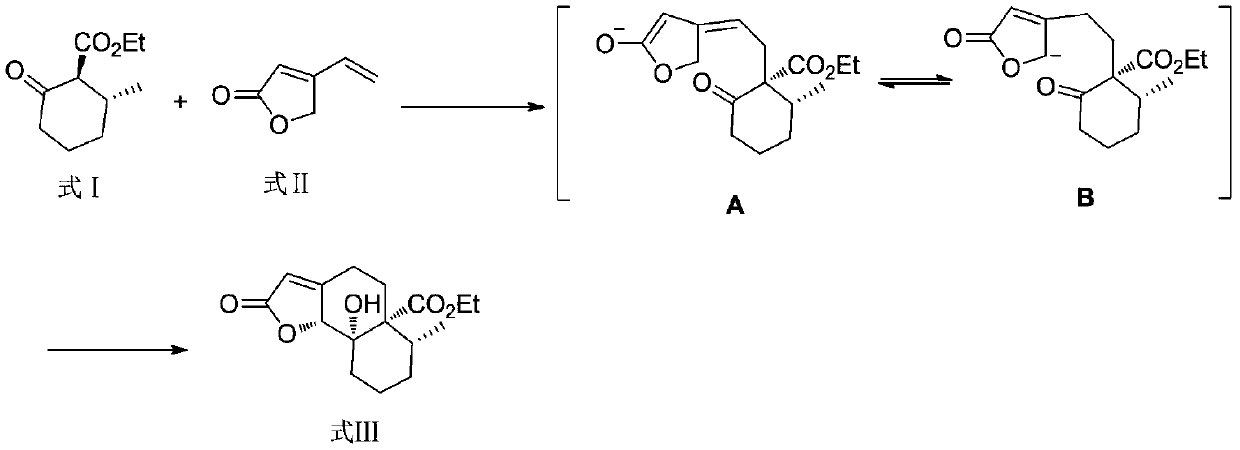
[0028] The preparation method of the compound of formula III of the present invention is to use the compound of formula I as the starting material, and undergo 1,6-conjugate addition / aldol series reaction with the compound of formula II to obtain the target product in one step. The reaction route is as follows:
[0029]
Embodiment 1
[0030] Embodiment 1: the synthesis of formula III compound
[0031] Compound 1 (368 mg, 2 mmol) was dissolved in THF (3 mL), NaH (40 mg, 1 mmol) was added, and after 15 minutes, a THF (2 mL) solution of compound 2 (110 mg, 1 mmol) was added, reacted at room temperature for 3 hours, and saturated chloride was added The reaction was quenched with ammonium aqueous solution, extracted with ethyl acetate, concentrated, and separated on a silica gel column to obtain compound 3 (208 mg, yield 70%).
[0032] 1 H NMR (500MHz, CDCl 3 )δ5.78(s,1H),5.19(s,1H),4.18(dd,J=7.1,5.2Hz,2H),2.81–2.64(m,2H),2.41(s,2H),2.32–2.14 (m,2H),2.07–1.91(m,2H),1.87–1.60(m,3H),1.36–1.29(m,3H),0.98(d,J=6.7Hz,3H).
[0033] 13 C NMR (101MHz, CDCl 3 )δ173.73, 172.31, 168.75, 113.18, 80.35, 76.81, 60.64, 54.94, 32.10, 31.65, 29.59, 27.33, 23.06, 21.94, 17.03, 14.12
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com