A post-treatment method of mesotrione reaction product
A technology for mesotrione and reaction products, which is applied in the field of post-treatment of mesotrione reaction products, can solve problems such as dissolution loss, low yield, and interference with the system environment, and achieve less three wastes, high product yield, and high purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
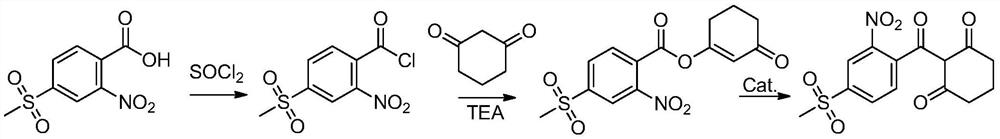
[0042] The preparation method of the above-mentioned mesotrione reaction product is generally carried out according to the synthetic method of CN85109771A, with 4-thiamphenicol-2-nitrobenzoic acid as raw material, dichloroethane as solvent, acylated with thionyl chloride , and then use triethylamine as an acid-binding agent to esterify with cyclohexanedione, and finally use acetone cyanohydrin as a catalyst for transposition to obtain the reaction product of mesotrione.
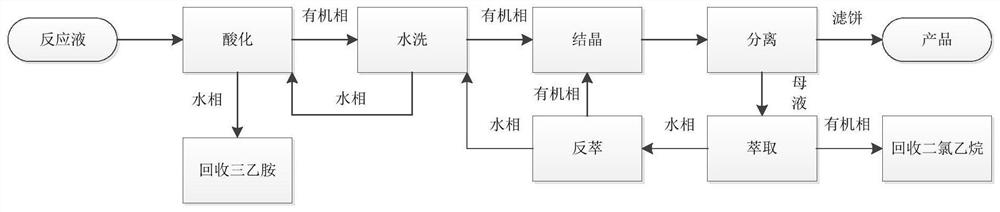
[0043] In the prior art, when the mesotrione reaction product obtained by the above synthesis method is post-treated, alkali is generally directly added to the mesotrione reaction product, and the mesotrione dissolved in the organic phase is extracted into the water In this way, follow-up purification is carried out, although the problem of removing organic reagents is avoided, and the product can be obtained by subsequent acid adjustment, but this method reduces the yield of the product, and the product canno...
preparation example
[0073] The preparation method of mesotrione reaction product solution is:
[0074] (1) Acylation: 2-nitro-4-thiamphenicol benzoic acid is subjected to an acylation reaction with the acylating agent thionyl chloride to obtain an acylation reaction liquid, and the temperature of the acylation reaction is 10-80° C.;
[0075] (2) Esterification: add an acid-binding agent and 1,3-cyclohexanedione to the acylation reaction solution to carry out esterification reaction to obtain an esterification reaction solution, the temperature of the esterification reaction is 0-65°C , the acid-binding agent is triethylamine;
[0076] (3) Rearrangement: add rearrangement catalyst acetone cyanohydrin to the esterification reaction solution to carry out rearrangement reaction, and transposition to obtain mesotrione solution.
Embodiment 1
[0078] The aftertreatment method of mesotrione reaction product solution is:
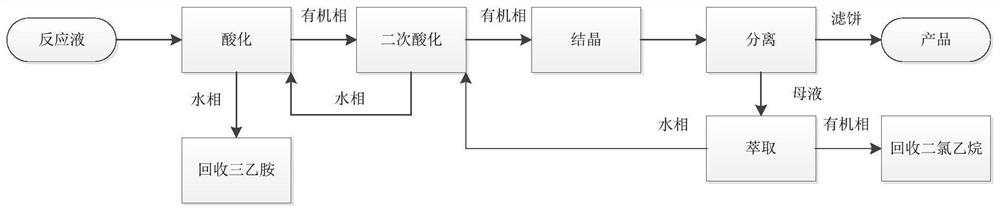
[0079] 1) Primary acidification: add water to the mesotrione reaction product solution obtained in the above preparation example, and add hydrochloric acid dropwise to adjust the pH value to 1-2, raise the temperature to 40-50°C, let stand to separate, and remove the water phase to recover triethylamine Amine, organic phase continues to carry out the purification of next step;
[0080] 2) Secondary acidification: adding potassium bicarbonate to the organic phase to extract water, adding hydrochloric acid dropwise to adjust the pH value to 1-2 for secondary acidification, raising the temperature to 40-50°C, standing for stratification, and recycling the water phase to the next batch an acidification;
[0081] 3) Crystallization, the organic phase is cooled to 0°C to crystallize;
[0082] 4) Separate and filter, filter the crystalline slurry at 0°C to obtain the product, add the dichloroethane mothe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com