Catalyst for preparing corresponding olefins through dehydrogenation of low-carbon alkanes and application of catalyst
A technology of low-carbon alkanes and catalysts, which is applied in the field of catalysts for preparing corresponding olefins from the dehydrogenation of low-carbon alkanes. It can solve the problems of irreversible loss of catalysts, reduction of single-pass conversion rate, metal sintering loss, etc., achieve good industrial application prospects, and maintain catalyst activity. , the effect of suppressing the problem of sintering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
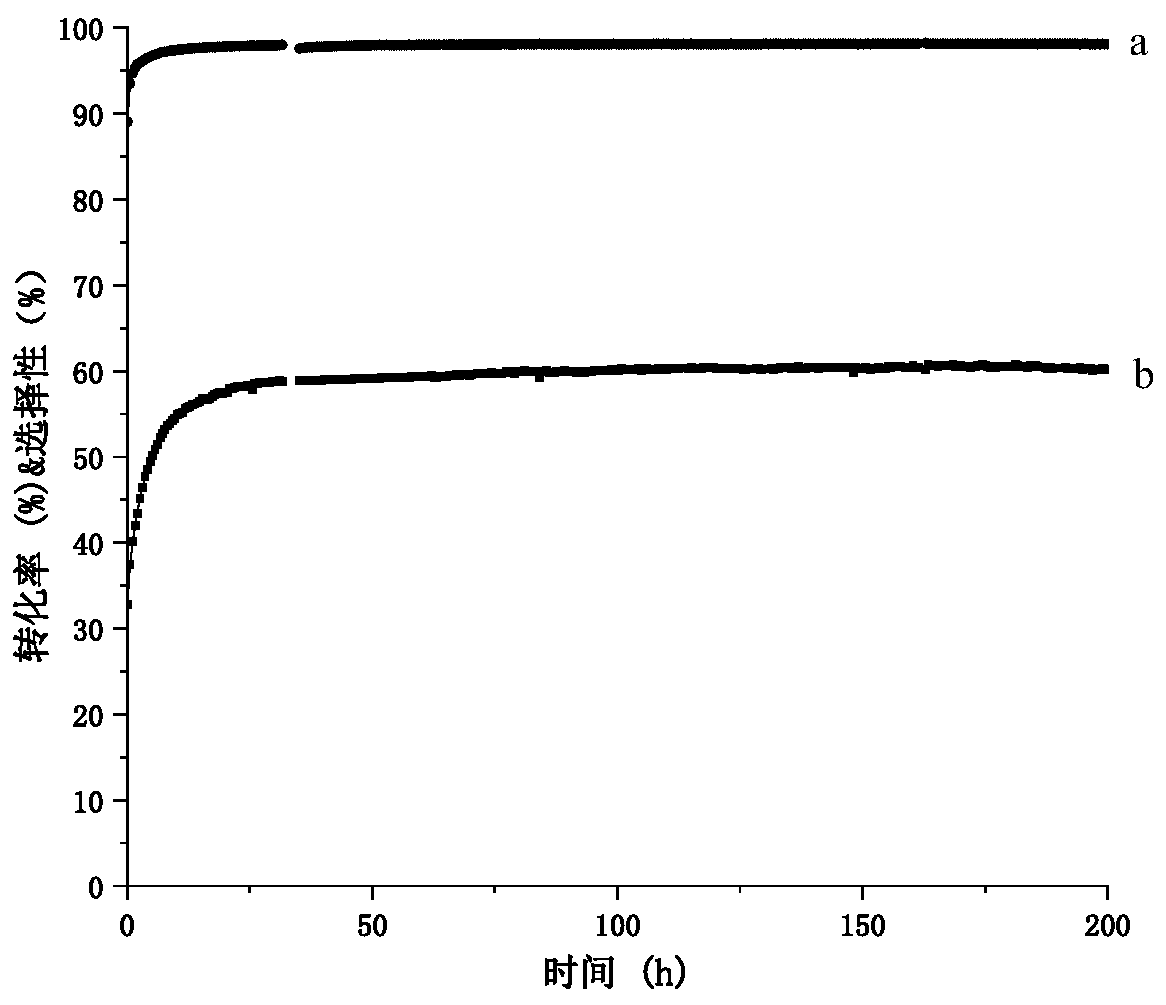
[0026] Take 0.22g 0.4%Pt-2%Cu@MOR (SAR=20) catalyst of 30~60 meshes, put it into a quartz reaction tube (inner diameter 10mm), at a flow rate of 15ml min -1 Under nitrogen atmosphere, at 10°C min -1The heating rate was heated from room temperature to 550 °C and kept for 30 min. The catalytic reaction is carried out in a fixed-bed reactor at normal pressure, and the reaction conditions are: the reaction gas is butane and nitrogen, and the flow rate is 30ml min -1 , 80ml min -1 , the reaction temperature is 550° C., and the reaction pressure is normal pressure. The reaction products were analyzed online by gas chromatography. The catalytic performance (T = 550°C) of the 0.4%Pt-2%Cu@MOR catalyst is shown in Table 1.
[0027] Table 1
[0028] Butane conversion (%) 64 Butene selectivity (%) 99.1 Methane selectivity (%) 0.3 Ethane selectivity (%) 0.6 Ethylene selectivity (%) 0
Embodiment 2
[0030] Take 0.22g 0.8%Pd-1.6%Ga@MCM-22 (SAR=30) catalyst of 30~60 meshes, put it into a quartz reaction tube (inner diameter 10mm), at a flow rate of 15ml min -1 Under nitrogen atmosphere, at 10°C min -1 The heating rate was heated from room temperature to 600 °C and kept for 30 min. The catalytic reaction was carried out in a fixed-bed reactor at normal pressure, and the reaction conditions were: the reaction gas was ethane and argon, and the flow rates were 5ml min -1 , 15ml min -1 , the reaction temperature is 750°C and normal pressure. The reaction products were analyzed online by gas chromatography. The catalytic performance (T=750°C) of 0.8%Pd-1.6%Ga@MCM-22 catalyst is shown in Table 2.
[0031] Table 2
[0032] Ethane conversion rate (%) 30 Ethylene selectivity (%) 85.4 Methane selectivity (%) 14.5
Embodiment 3
[0034] Take 0.22g 0.5%Rh-2%Zn@S-1 catalyst of 30~60 meshes, put it into a quartz reaction tube (inner diameter 10mm), at a flow rate of 15ml min -1 Under nitrogen atmosphere, at 10°C min -1 The heating rate was heated from room temperature to 600 °C and kept for 30 min. The catalytic reaction is carried out in a fixed-bed reactor at normal pressure, and the reaction conditions are as follows: the reaction gas is propane and helium, and the flow rate is 20ml min -1 、60ml min -1 , the reaction temperature is 600°C, and the reactor is at normal pressure. The reaction products were analyzed online by gas chromatography. The catalytic performance of the 0.5%Rh-2%Zn@S-1 catalyst (T = 600°C) is shown in Table 3.
[0035] table 3
[0036] Propane Conversion (%) 65 Propylene selectivity (%) 98.4 Methane selectivity (%) 0.5 Ethane selectivity (%) 1.0 Ethylene selectivity (%) 0.1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com