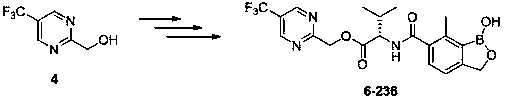
Synthesis method of 2-hydroxymethyl-5-trifluoromethylpyrimidine
A technology for the synthesis of trifluoromethylpyrimidine and its synthesis method, which is applied in the field of synthesis of 2-hydroxymethyl-5-trifluoromethylpyrimidine, and can solve the industrial synthesis method of 2-hydroxymethyl-5-trifluoromethylpyrimidine There are no public reports and other problems, and the effect of cheap raw materials, unique reaction circuit and mild reaction conditions is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] step 1:
[0011] Add chloromethyl pivalate (40 g, 266 mmol) and acetonitrile (80 mL) to a 250 mL single-necked bottle, slowly add anhydrous sodium iodide (72 g, 480 mmol), under argon protection, room temperature ( about 25°C) to react overnight. Add water (400 mL) and dichloromethane (400 mL), stir for 5 min, and separate the layers. The organic phase is washed with 2% sodium thiosulfate (200 mL), dried over anhydrous sodium sulfate, and spin-dried ( below 40°C). Vacuum distillation (oil temperature 50°C) gave a colorless liquid, Compound 1 (55 g, 227 mmol, 85%).
[0012] Step 2:
[0013] Under the protection of argon, cool down in ice water, add zinc powder (27.2 g, 0.42 mol) and anhydrous tetrahydrofuran (120 mL) into a 500 mL three-necked flask, stir, add 10 drops of 1,2-dibromoethane, trimethyl Chlorosilane 10 drops, stirred for 5 minutes. Add the tetrahydrofuran solution (60 mL) of compound 1 (51.5 g, 0.21 mol) dropwise. After adding about 1 / 5 of the volume, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com