Tetraphenylethylene compound containing sialic acid glycosyl unit, preparation method and application
A technology of sialic acid sugar and tetraphenylethylene, which is applied in the field of fluorescent probe research to achieve the effects of enhanced fluorescence intensity, improved detection sensitivity, good photostability and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
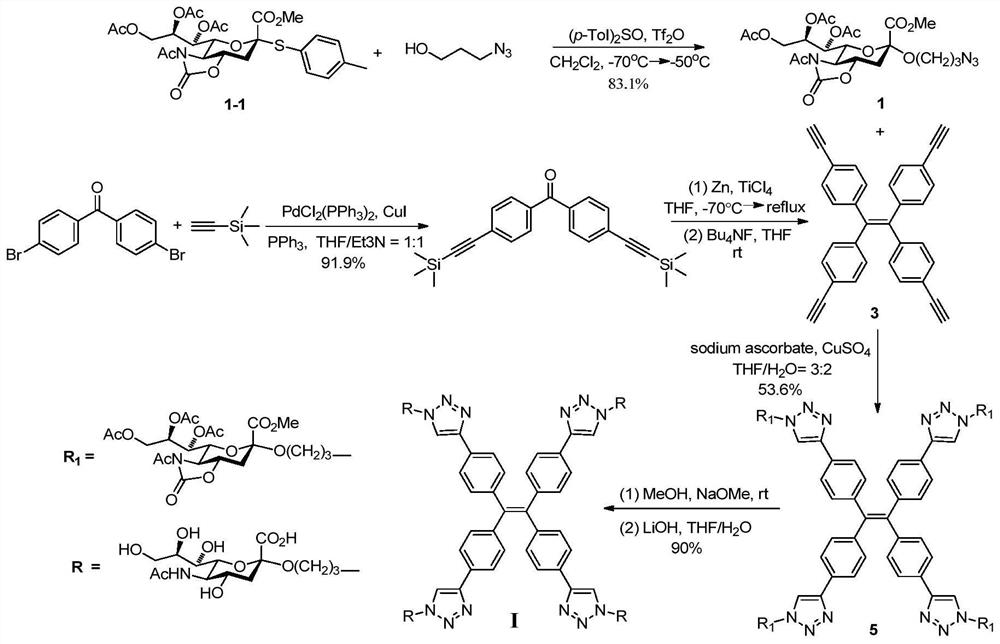
[0027] Synthesis of Compounds of Formula I.
[0028] attached figure 1 A synthetic route diagram for the compound of formula I is shown.
[0029] Step A: Methyl N-acetyl-7,8,9-tri-O-acetyl-5-N,4-O-oxazolidinone-2-(3-azidopropoxy)sialate (compound 1)
[0030] Molecular sieves are added to the long reaction flask, heated and burned to remove water, and after cooling, add N-acetyl-7,8,9-tri-O-acetyl-5-N,4-O-oxazolidinone-protected sialic acid p-methylglucosinolate (compound 1-1, 136.4mg, 0.235mmol), p-tolylsulfoxide (108.1mg, 0.469mmol), seal the reaction tube, replace the gas with argon 3 times, add anhydrous dichloromethane (8 mL). The reaction tube was stirred at -70°C for 15 minutes, trifluoromethanesulfonic anhydride (47 μL) was added with a micro syringe, stirred at -70°C for 30 minutes, 3-azido-1 propanol (35.6 mg, 0.352 mmol) was added Dichloromethane (2 mL) solution. The reaction was stirred at -70°C for 2 hours and then at -50°C for 2 hours. Triethylamine (0.2 ...
Embodiment 2
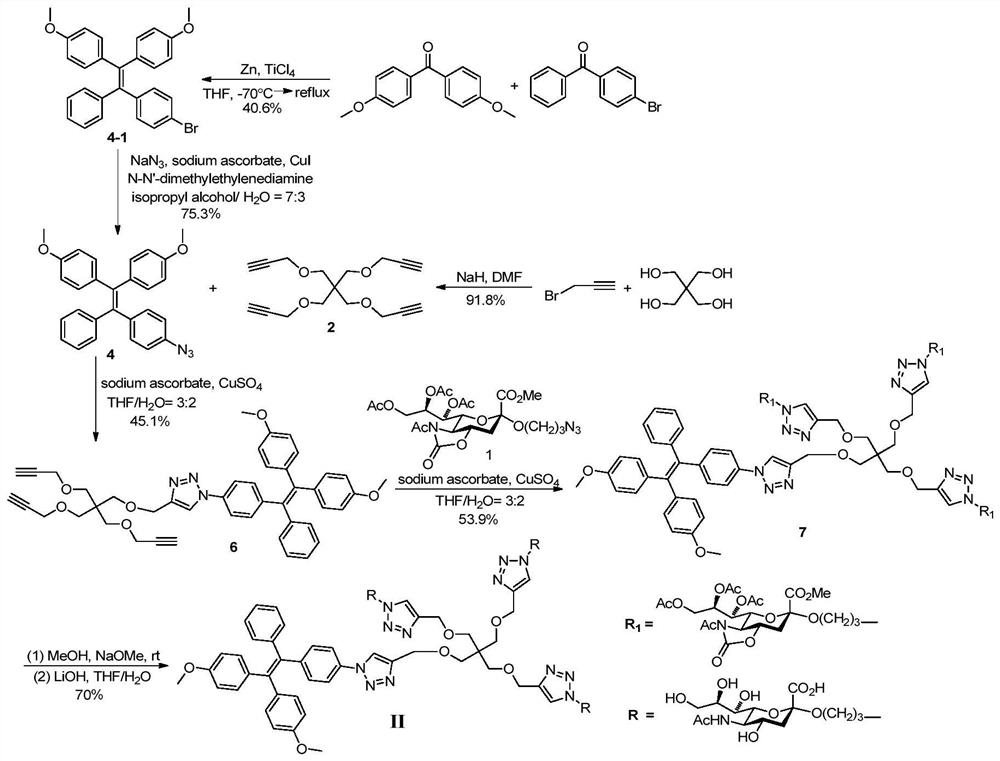
[0038] Synthesis of Compounds of Formula II.
[0039] attached figure 2 A synthetic route diagram for the compound of formula II is shown.
[0040] Step A: Methyl N-acetyl-7,8,9-tri-O-acetyl-5-N,4-O-oxazolidinone-2-(3-azidopropoxy)sialate (Compound 1), prepared according to the method described in step A of Example 1.
[0041] Step B: Tetrapropargylpentaerythritol (compound 2)
[0042] Pentaerythritol (1.3615g, 10mmol) was dissolved in N,N-dimethylformamide (35mL), oil-dispersed sodium hydride (120mmol) was slowly added in an ice-salt bath, and after stirring for 30 minutes, propargyl Bromine (6 mL). After the reaction solution was reacted at 40° C. for 2.5 hours, 3 mL of propargyl bromide was added. The reaction solution was placed in an oil bath at 50°C for 16 hours. After cooling to room temperature, the reaction was quenched by adding water and extracted twice with ethyl acetate. The organic phase was dried with anhydrous sodium sulfate, filtered, and concentrated. ...
Embodiment 3
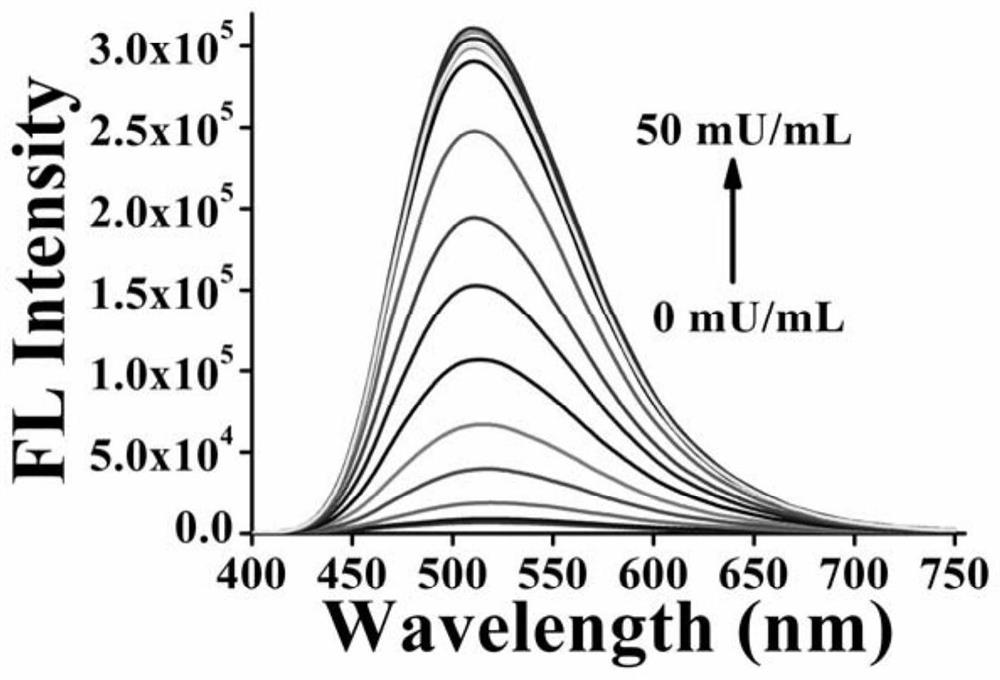
[0053] Formula I compound detection sialidase performance test
[0054] 1) Principle: sialidase can act on natural or synthetic sialyl glycoside substrates. After the target compound reacts with sialidase, the glycosidic bond is broken, the solubilizing sialic acid unit leaves, the solubility of the molecule decreases, and aggregation occurs induced luminescence. The activity of sialidase can be detected sensitively through the change of fluorescence.
[0055] 2) Test material:
[0056] Commercially available sialidase (the sialidase is produced by Clostridium perfringens, one unit of activity is defined as: one unit of this enzyme is produced at pH 5.0 and 37°C with sialic acid (2→3) lactose as the substrate The product can release 1.0μmol N-acetylneuraminic acid per minute.
[0057] Phosphate buffer solution: pH 7.1.
[0058] Compound of formula I: 10 mM stock solution.
[0059] 3) Test method:
[0060] Different concentrations of sialidase and the compound of formula ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com