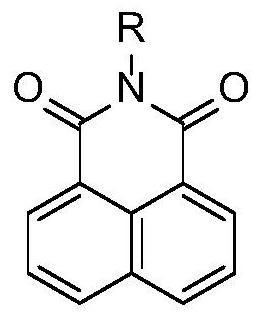
Application of a two-photon fluorescent probe for detecting cytochrome oxidase cyp3a4
A cytochrome and fluorescent probe technology, applied in the application field of two-photon fluorescent probes, can solve the problems of differences in the distribution of cell structures, the role of the central nervous system is not yet clear, etc., and achieve good specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
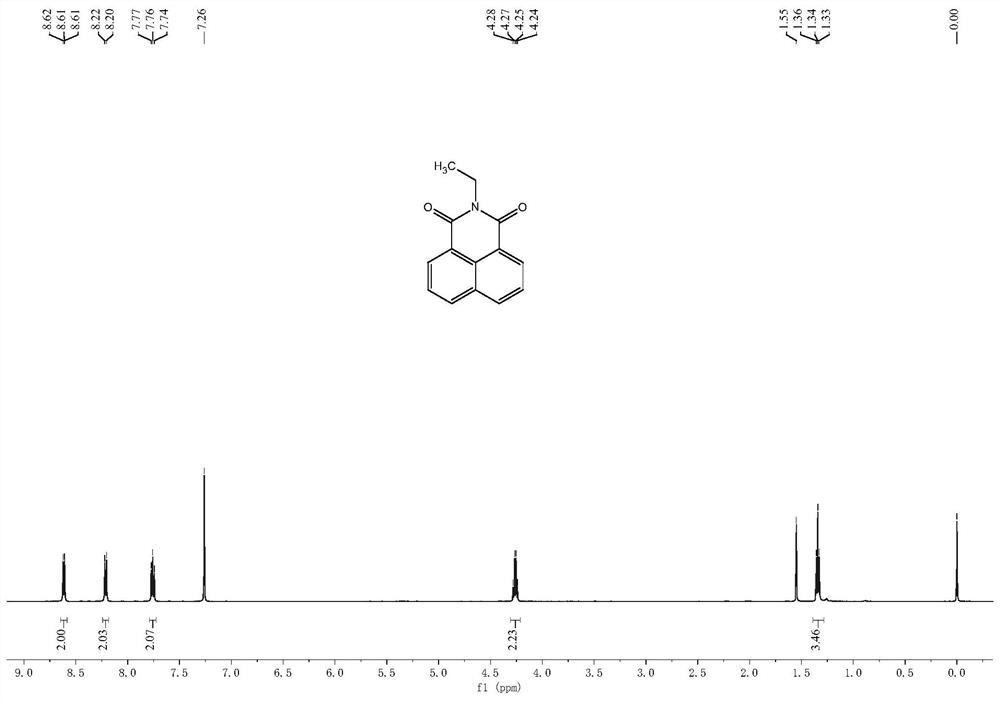
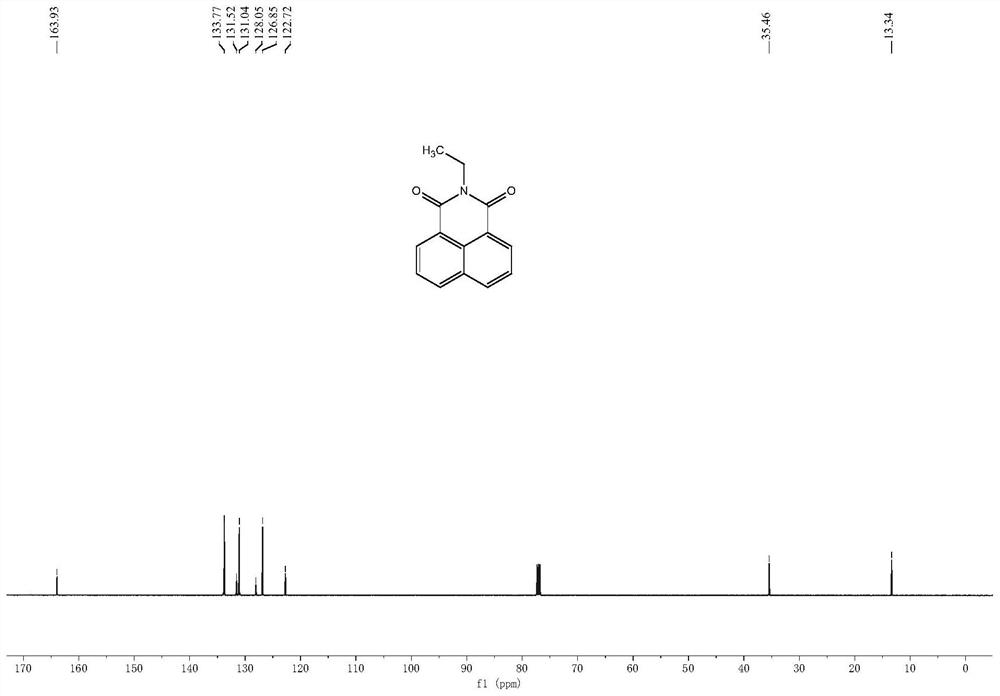
[0041] Example 1. Synthesis of N-ethyl-1,8-naphthalimide
[0042] Add 5 mmol of 1,8-naphthalene anhydride and 5.5 mmol of ethylamine to 30 ml of ethanol, heat to reflux for 12 hours, cool, filter the solid, rinse the filter cake with ethanol, and dry to obtain a milky white solid (yield 63% ).
[0043]
[0044] 1 H NMR (500 MHz, CDCl 3) δ 8.65 – 8.58 (m, 2H), 8.21 (d, J = 8.3 Hz,2H), 7.79 – 7.73 (m, 2H), 4.26 (q, J = 7.1 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H). 13 C NMR (125 MHz, CDCl 3 ) δ 163.93, 133.77, 131.52, 131.04, 128.05, 126.85, 122.72, 35.46, 13.34. HRMS calcd for [M+H] + 226.0863, found 226.0683.
[0045] Note: The compound N-ethyl-1,8-naphthalimide 1 H-NMR spectrum, 13 C-NMR spectra and high-resolution mass spectrograms such as figure 2 , 3 , 4 shown.
Embodiment 2
[0046] Example 2. Synthesis of N-benzyl-1,8-naphthalimide
[0047] Add 5 mmol of 1,8-naphthalene anhydride and 5.5 mmol of benzylmethylamine into 30 ml of ethanol, heat to reflux for 12 hours, cool, filter the solid, rinse the filter cake with ethanol, and dry to obtain a light milky white solid (yield 55%).
[0048]
[0049] 1 H NMR (500 MHz, CDCl 3 ) δ 8.61 (dd, J = 7.3, 0.9 Hz, 2H), 8.20 (d, J =8.3 Hz, 2H), 7.75 (t, J = 7.8 Hz, 2H), 7.55 (d, J = 7.2 Hz, 2H), 7.30 (t, J =7.4 Hz, 2H), 7.24 (d, J = 7.3 Hz, 1H), 5.39 (s, 2H). 13 C NMR (125 MHz, CDCl 3 )δ 164.13, 137.35, 133.96, 131.47, 131.32, 129.08, 128.45, 128.03, 127.52,126.88, 122.53, 43.54. HRMS calcd for [M+H] + 288.1019, found 288.1024.
[0050] Note: N-benzyl-1,8-naphthalimide 1 H-NMR spectrum, 13 C-NMR spectra and high-resolution mass spectrograms such as Figure 5 , 6 , 7 shown.
Embodiment 3
[0051] Example 3. In vitro determination of the selectivity of human recombinant CYP single enzyme
[0052] (1) Prepare 90 µL CYP metabolic reaction system in advance, including PBS buffer (100 mM) at pH 7.4, recombinant human CYP individual enzymes, and the final concentration of N-ethyl-1,8-naphthalimide is 10 μM, at 37 o Pre-incubation with shaking for 3 minutes under C condition;
[0053] (2) Add 10 µL of 10 mM NADP to the reaction system + initial reaction;
[0054] (3) After 40 minutes, add 50 µL of glacial acetonitrile and shake vigorously to terminate the reaction;
[0055] (4) Use a high-speed refrigerated centrifuge at 4 o C, 20,000 × g Under the conditions of high-speed centrifugation for 20 minutes, the supernatant was taken for fluorescence detection (E x = 450 nm, E m = 558 nm); the selectivity of recombinant human CYP3A4 enzyme is about 29 times higher than that of other single enzymes ( Figure 8 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com