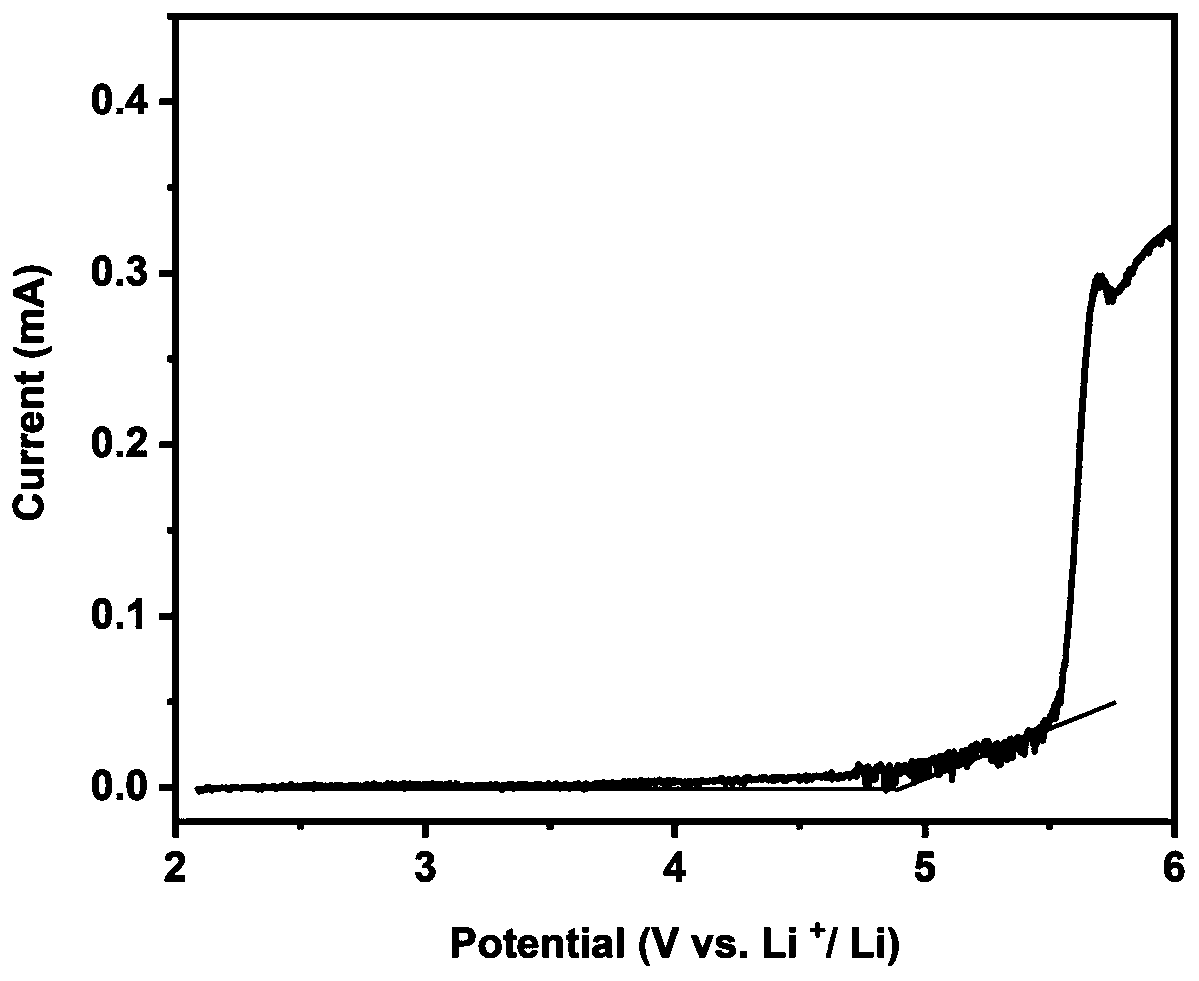
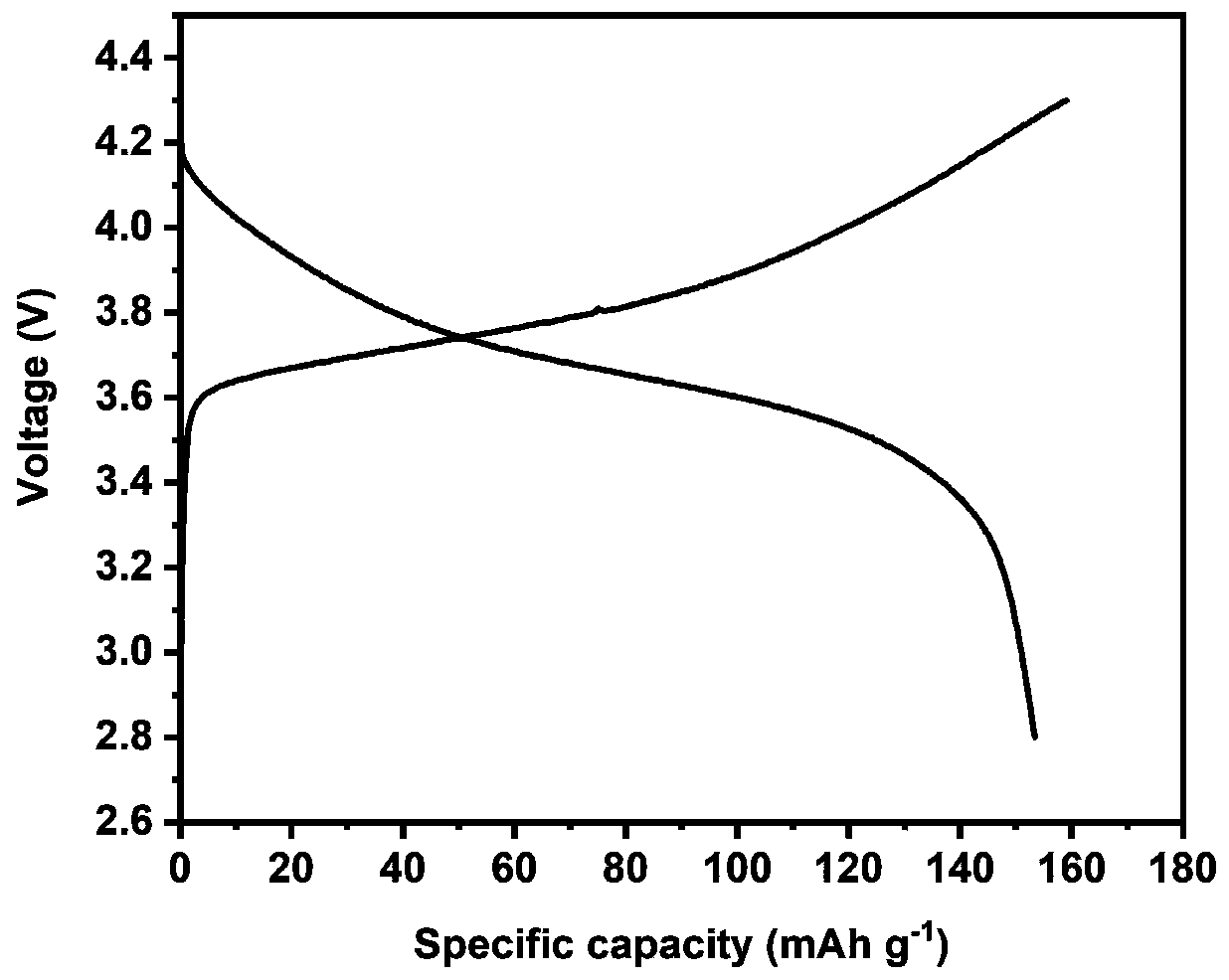
A kind of all-solid electrolyte and its preparation method and a kind of lithium battery
An electrolyte and all-solid-state technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems such as low ion conductivity, low ion migration number, and low energy density, and achieve improved ion conductivity and high ion conductivity , the effect of wide electrochemical window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] In the present invention, the polycaprolactone-based block copolymer is preferably a self-made polycaprolactone-based block copolymer. In the present invention, the preparation method of the polycaprolactone-based block copolymer preferably comprises the following steps:
[0032] Mixing caprolactone, a hydroxyl-terminated polymer, a catalyst and a solvent to react to obtain a product system;
[0033] The obtained product system is dissolved in a polar solvent, then precipitated and purified with a non-polar solvent, and dried to obtain the polycaprolactone-based block copolymer.
[0034] In the invention, caprolactone, a hydroxyl-terminated polymer, a catalyst and a solvent are mixed and then reacted to obtain a product system. In the present invention, the hydroxyl-terminated polymer is specifically one or more of hydroxyl-terminated polytrimethylene carbonate, hydroxyl-terminated polyethylene oxide, and hydroxyl-terminated polypropylene carbonate, so Said solvent is...
Embodiment 1
[0055] Step 1: Dissolve 3g caprolactone and 1g hydroxyl-terminated polytrimethylene carbonate in 50mL toluene, and divalent tin compound Sn(Oct) 2 Add wherein according to 0.5% of the total mass.
[0056] Step 2: The above mixture was heated to 100° C. and stirred for 12 h.
[0057] Step 3: The crude product after the reaction was dissolved in tetrahydrofuran, and purified by reprecipitation with n-hexane.
[0058] Step 4: drying the purified product in a high-temperature vacuum oven at 60° C. for 15 hours to obtain polycaprolactone-polytrimethylene carbonate-polycaprolactone.
[0059] Step five: the synthesized polycaprolactone-polytrimethylene carbonate-polycaprolactone and LiPF 6 Added in anhydrous acetonitrile and stirred until uniform and poured on the porous cellulose diaphragm, the synthesized polycaprolactone-polytrimethylene carbonate-polycaprolactone and LiPF 6 The mass ratio of the anhydrous acetonitrile is 9:1, and the mass ratio of the volume of anhydrous aceto...
Embodiment 2
[0069] Step 1: Dissolve 3g caprolactone and 2g hydroxyl-terminated polypropylene carbonate in 100mL xylene, and divalent tin compound Sn(Oct) 2 Add wherein according to 0.5% of the total mass.
[0070] Step 2: The above mixture was heated to 120° C. and stirred for 48 hours.
[0071] Step 3: The crude product after the reaction was dissolved in tetrahydrofuran, and purified by reprecipitation with petroleum ether.
[0072] Step 4: drying the purified product in a high-temperature vacuum oven at 80° C. for 18 hours to obtain polycaprolactone-polypropylene carbonate-polycaprolactone.
[0073] Step 5: Add the synthesized polycaprolactone-polypropylene carbonate-polycaprolactone and LiTFSI into anhydrous acetonitrile and stir until evenly poured on the glass fiber diaphragm, the synthesized polycaprolactone-poly The mass ratio of propylene carbonate-polycaprolactone and LiTFSI is 8:2, and the mass ratio of the volume of anhydrous acetonitrile and polycaprolactone-polypropylene c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com