Preparation method and application of hybrid nano-tumor vaccine
A tumor vaccine and nanotechnology, applied in the field of preparation of hybrid nano-tumor vaccine, can solve the problems of low activation efficiency of immune response, increase anti-tumor immune effect, low immunogenicity, etc., achieve no immune response and inflammatory response, and facilitate the Large-scale production, good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of hybrid nano tumor vaccine
[0032] The specific preparation method is as follows:
[0033] (1) Dissolve 20 mg of sterile Melittin-RADA32 powder in 1 ml of sterile deionized water to obtain a Melittin-RADA32 solution with a concentration of 20 mg / ml for use.
[0034] (2) Mix 8 mg / ml CpG-ODN and 40 μg / ml lysis aqueous solution in equal proportions.
[0035] (3) The Melittin-RADA32 solution in the step (1) is fully mixed with the solution in the step (2), and an aqueous sodium chloride solution (final concentration is 0.9wt%) is added to the mixed solution to obtain the Melittin-RADA32 / CpG / lysis nano vaccine, wherein the final concentrations of Melittin-RADA32, CpG-ODN, and lysis are 10mg / ml, 2mg / ml and 10μg / ml respectively. Place the mixture at 4°C for 2 hours to form a gel, as figure 1 As shown, the prepared nano-vaccine is a milky white gel under the naked eye.
[0036] The physical and chemical properties of the MLC self-assembled ...
Embodiment 2
[0037] Example 2: Assessing the security of MLC
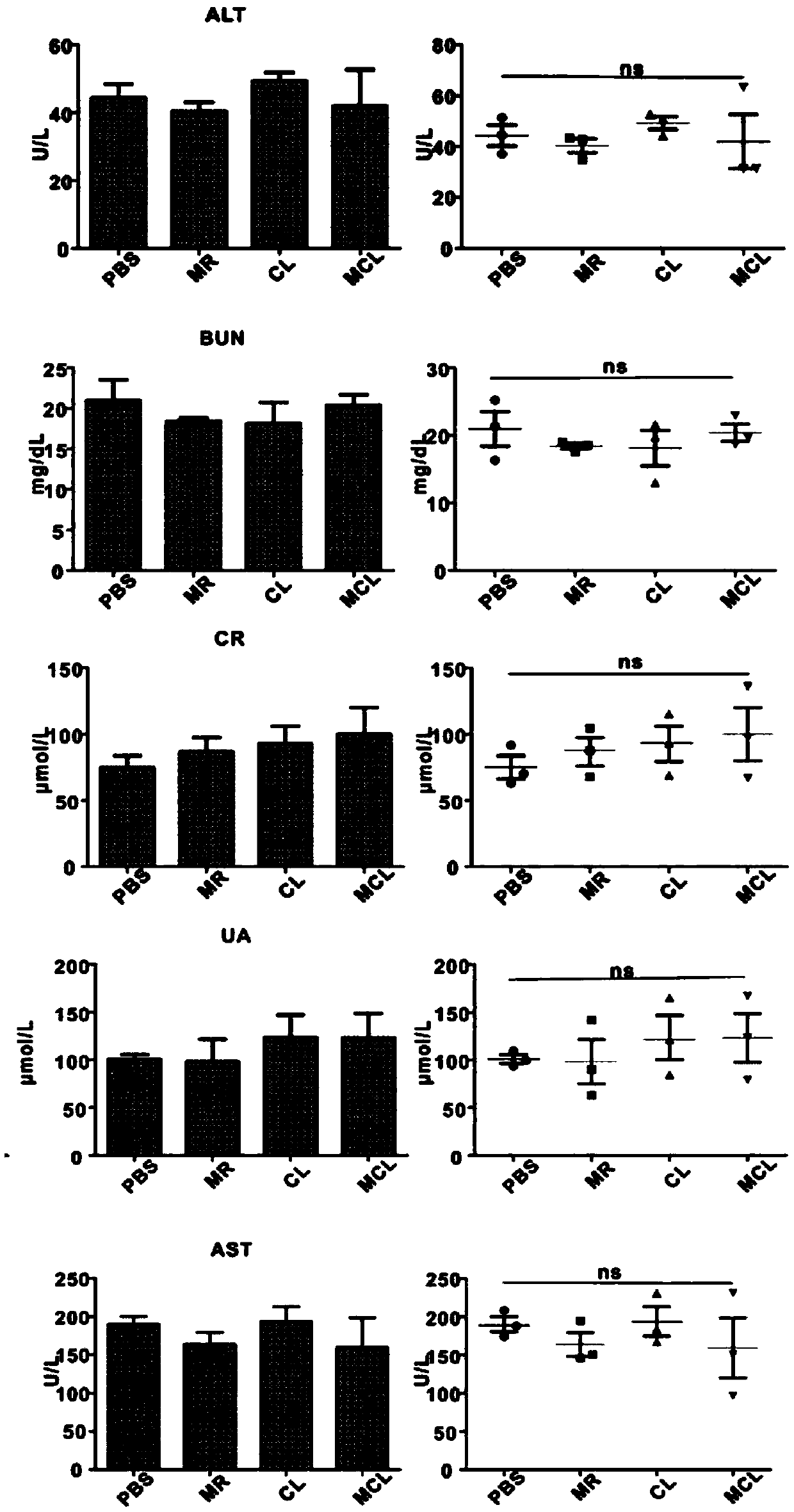
[0038] Take B16-luc10 cells in the logarithmic growth phase, adjust the cell concentration to 1x10*7 / ml, and inoculate them subcutaneously into the right hind limb of C57 mice, with an inoculation volume of 50 μl, until the tumor grows to 40-50mm 3, the mice were randomly divided into PBS group, Melittin-RADA32 group (MR group), CpG+Lysis group (CL group), MCL group (Example 1MLC self-assembled hybrid nanovaccine), with 5-6 mice in each group. The method of subcutaneous injection at the base of the tail + foot pad was used for injection, and the dose of each injection was 50 μl. Dosing once every other day, a total of 3 administrations. When the tumor volume of the PBS control group reached 1000mm 3 Afterwards, the hearts, livers, spleens, lungs, and kidneys of the mice were taken for HE staining, and the blood of the mice in each group was taken for biochemical detection. image 3 As shown, AST, ALT, BUN, Cr, and UA were al...
Embodiment 3
[0039] Example 3: Evaluation of MCL's ability to activate DC cells
[0040] Bone marrow stem cells were obtained from the femur and tibia of 6-8-week-old male C57 mice, cultured in 1640 medium containing M-CSF for 5 days to obtain immature DC cells, and then planted in 24-well plates, divided into PBS group, Melittin- RADA32 group (MR group), CpG+Lysis group (CL group), MCL group (Example 1MLC self-assembled hybrid nanovaccine) and lipopolysaccharide LPS (positive control), each group takes 40 μ l of the corresponding solution and immature DC cells Co-incubated to stimulate the maturation of DC cells, and after 24 hours, use flow cytometry to detect the activation of CD11c, CD80, and CD86 cells (such as Figure 4 shown), the experimental results showed that the novel hybrid nanovaccine MCL with stable loading could promote the maturation of DCs.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com