Acid pH probe compound and production method thereof
A compound and acidic technology, applied in the field of fluorescent probes, can solve the problems of loss of structure and unsatisfactory stability, and achieve the effect of stable structure, difficult hydrolysis and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Synthesis of acidic pH fluorescent probe.
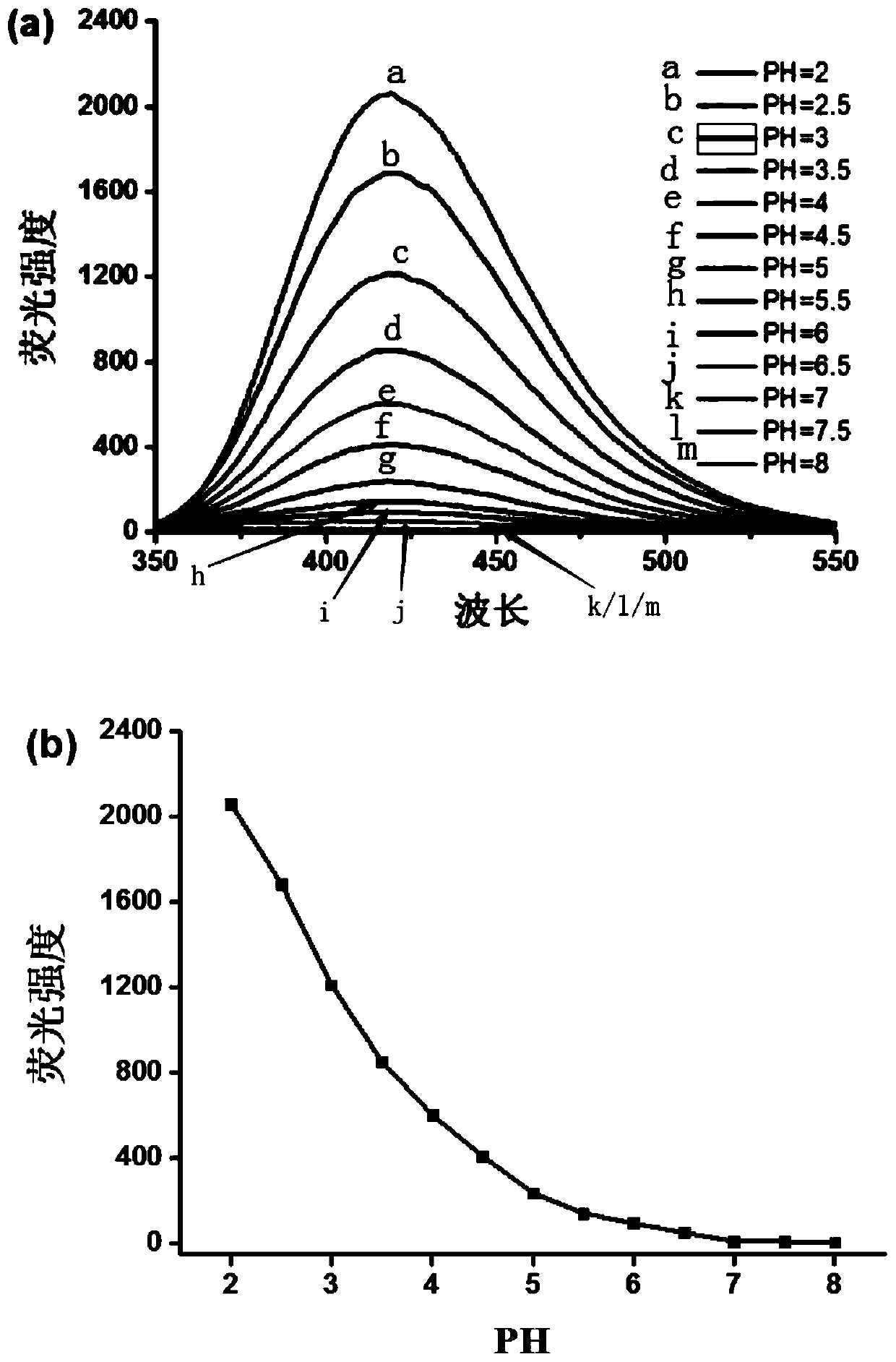
[0045] The acidic pH fluorescent probe compound has the molecular structural formula as the aforementioned formula I.
[0046] The preparation method steps are as follows:
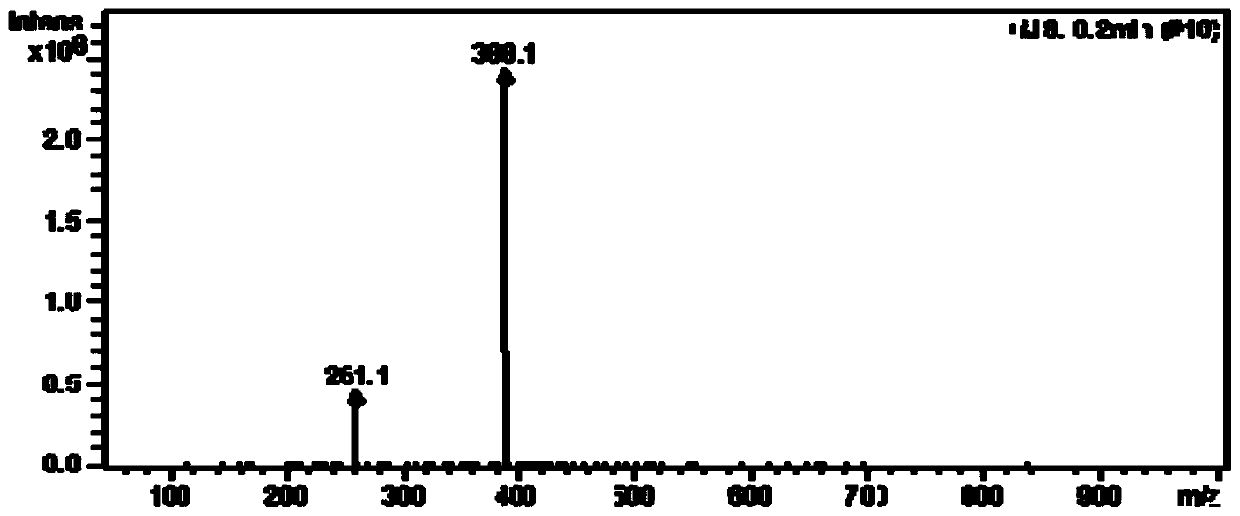
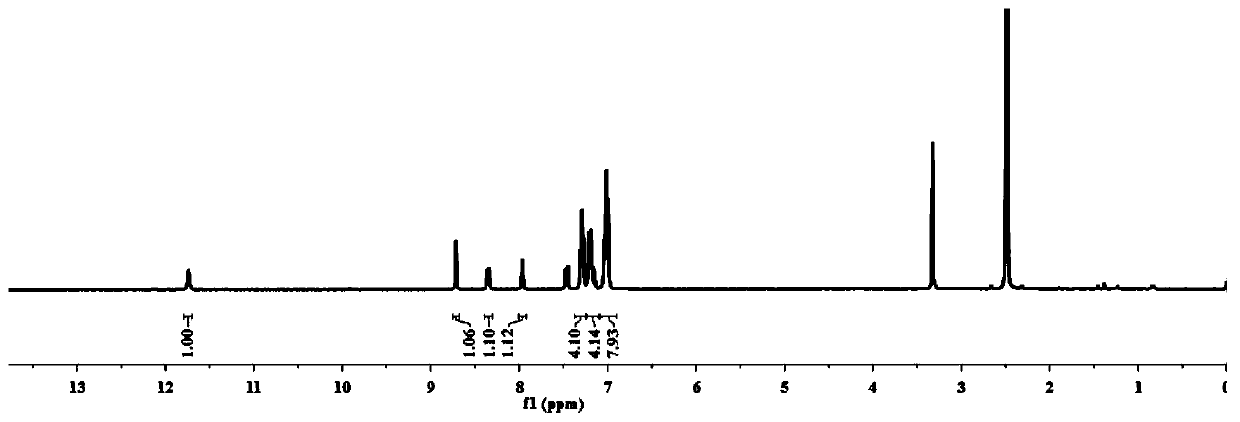
[0047] Dissolve 5-indole formaldehyde and 4-aminotriphenylamine in ethanol solvent, add two drops of glacial acetic acid, reflux at 60-70°C for 5-6h, cool to room temperature, filter with suction, wash with absolute ethanol, and dry to obtain pH probe compound. 1 H NMR (400MHz, DMSO) δ11.74(s,1H),8.72(s,1H),8.35(d,J=7.7Hz,1H),7.97(d,J=1.9Hz,1H),7.51–7.40 (m,1H),7.29(t,J=7.7Hz,4H),7.23–7.10(m,4H),7.02(t,J=9.3Hz,8H).
Embodiment 2
[0048] The preparation of embodiment 2 compound
[0049] a. Preparation of 4-nitrotriphenylamine
[0050] Slowly add triphenylamine and an appropriate amount of dichloromethane into a round bottom flask, slowly add concentrated nitric acid dropwise at 0°C, stir for 3 hours, remove dichloromethane by rotary evaporation, filter, wash with water, recrystallize with ethanol, and dry to obtain yellow solid. The molar ratio of triphenylamine to nitric acid is 1:3.
[0051] b. Preparation of 4-aminotriphenylamine
[0052] Dissolve 4-nitrotriphenylamine in ethanol solution, then add palladium carbon, and pass through hydrogen as a reducing agent. Then, react at 68°C for 6.5 hours, remove palladium carbon by filtration, remove ethanol by rotary evaporation, and remove palladium carbon by filtration. A certain amount of dichloromethane and methanol was selected for column chromatography, and the first point was collected to obtain a lavender solid with a yield of 61%.
[0053] The ...
Embodiment 3
[0057] The preparation of embodiment 3 compound
[0058] a. Preparation of 4-nitrotriphenylamine
[0059] Slowly add triphenylamine and an appropriate amount of dichloromethane into a round-bottomed flask, slowly add concentrated nitric acid dropwise at 0°C, stir for 2 hours, remove dichloromethane by rotary evaporation, filter, wash with water, recrystallize with ethanol, and dry to obtain yellow solid. The molar ratio of triphenylamine to nitric acid is 1:1.
[0060] b. Preparation of 4-aminotriphenylamine
[0061] Dissolve 4-nitrotriphenylamine in ethanol solution, then add palladium carbon, and pass through hydrogen as a reducing agent. Then, react at 60°C for 6 hours, remove palladium carbon by filtration, remove ethanol by rotary evaporation, and remove palladium carbon by filtration. A certain amount of dichloromethane and methanol was selected for column chromatography, and the first point was collected to obtain a lavender solid with a yield of 58%.
[0062] The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com