Lamb testicular sertoli cell immortalized cell line and its establishment method and application
A technology of Sertoli cells and immortalized cell lines, which is applied in the field of cell engineering, can solve the problems of complex manufacturing process, difficulty in obtaining profits for enterprises, and high production costs, and achieve the effects of reducing production costs, shortening the production cycle, and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
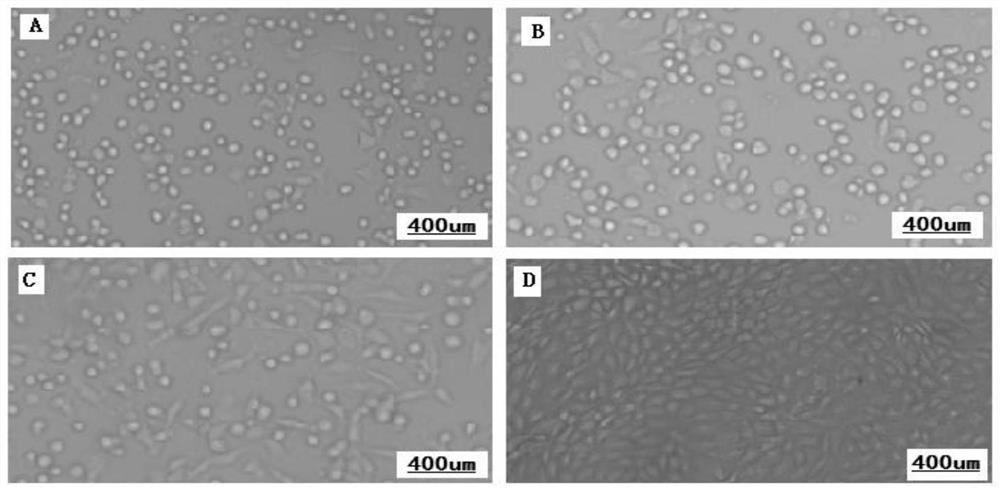
[0034] [Example 1] Isolation, purification and cultivation of lamb Sertoli cells (LSC)
[0035] 1.1 Isolation, purification and culture of lamb testis Sertoli cells (LSC)
[0036] Select healthy male lambs born to healthy ewes (whose physique is healthy and negative for foot-and-mouth disease, brucellosis, and tuberculosis), and collect the scrotum after slaughter (the root of the scrotum is ligated, and the outside of the scrotum is sterilized with 75% alcohol), in an incubator, for 2 hours sent back to the laboratory. The testicular parenchyma was aseptically collected, and the lamb testicular primary cells were prepared by digestion with collagenase and trypsin mixed enzymes (0.1wt% type IV collagenase (GIBCO), 0.25wt% trypsin (GIBCO)).
[0037] Using 6h differential speed attachment, 50mmol / L Tris_HCl, the cultured lamb testis primary cells were subjected to hypotonic treatment, 38.5°C high temperature culture, separation and purification to obtain lamb testis Sertoli cel...
Embodiment 2
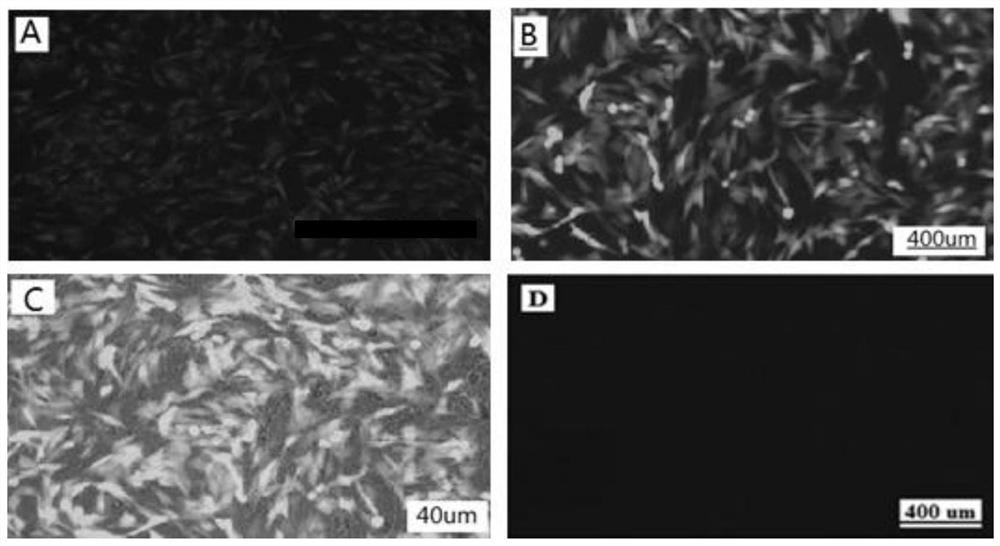
[0045] [Example 2] Establishment of Lamb Sertoli Cell Immortalized Cell Line (hTERT-LSC)
[0046] 2.1 Transfection of LSC cells with pCI-neo-hTERT plasmid
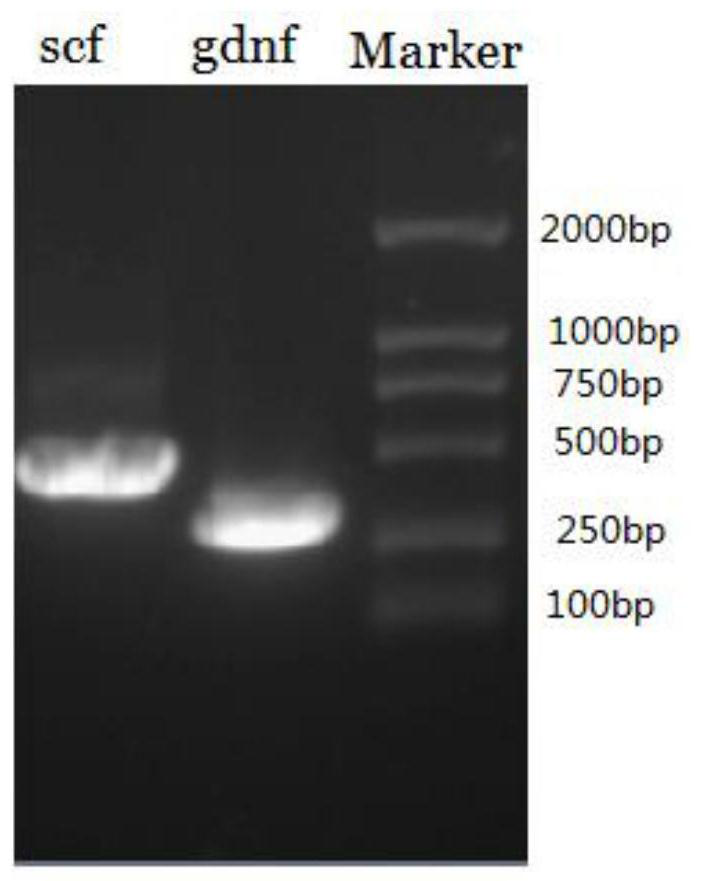
[0047] Example 1 was isolated and purified from lamb testis primary cells to obtain lamb testis Sertoli cells. According to the requirements of the Lipofectamine 2000Regent transfection instructions of Invitrogen Company, the eukaryotic expression plasmid pCI-neo-hTERT (vector map Such as Figure 4 (shown) was introduced into LSC (17th passage) to immortalize the LSC passage-limited cell line.
[0048] 2.2 Determination of optimal screening concentration of neomycin (G418)
[0049] Prepare LSC (17th passage) cells into a cell suspension at 1×10 5 cells / mL were inoculated into 24-well plates. Add G418 at a gradient of 50 μg / mL in the range of 100 μg / mL to 1 000 μg / mL, and add different concentrations of screening medium to each well. Four replicate wells were made for each gradient. According to the growth of the cell...
Embodiment 3
[0056] [Example 3] hTERT-LSC Biological Characteristic Measurement
[0057] 3.1 Growth characteristics of hTERT-LSC monoclonal cells and virus proliferation
[0058] Inoculate the hTERT-LSC monoclonal cell lines 1#~16# screened in Example 2 into 6-well plates respectively, and observe the seeding rate (referring to how many cells of the same volume can be inoculated from the primary cells in 1 bottle of cell bottle) Subculture in the flask), the time for the cells to grow into a monolayer; then inoculate the hTERT-LSC monoclonal cell lines 1#~16# that are covered with a monolayer with GTPV cell virulence (lgTCID 50 ≥6.0), observe the CPE produced by the virus on the cells, the results are shown in Table 2.
[0059] Table 2 Growth characteristics of hTERT-LSC monoclonal cells and virus proliferation
[0060]
[0061]It can be seen from Table 2 that the growth characteristics of each monoclonal cell line of hTERT-LSC are stable, the growth rate and cell division rate are ve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com