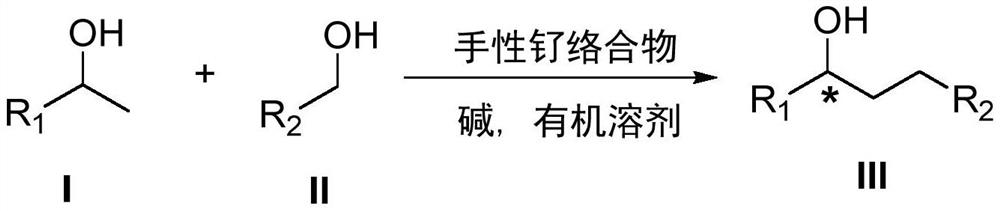
A kind of method of synthesizing chiral alcohol
A chiral alcohol and chiral technology, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve the problems of limited substrate scope and low atom economy, and achieves high reaction economic benefits and yields. High efficiency and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
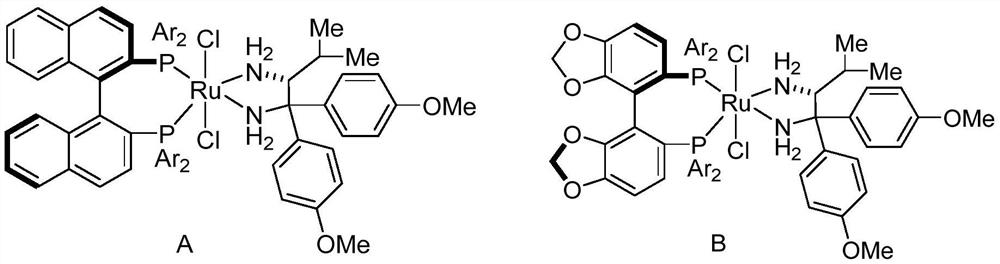
[0019] Under argon, 1-phenyl ethanol was 366 mg (3 mmol), 122 mg (1 mmol) of methylbenyl alcohol, 12 mg (0.01 mmol), tert-butoxide 112 mg (1 mmol) ) The mixture of dichloromethane is 20: 1 is a column, and the column chromatography is separated, and the structural formula is as follows:
[0020]
[0021] The yield of the above white solid was 64%, and the EE value of the EE value of high performance liquid chromatography was 86%, and its spectral data was: 1 HNMR (CDCL 3 400MHz) δ (PPM): 7.28 (D, J = 2Hz, 2H), 7.26 (S, 2H), 7.11 (S, 4H), 4.68 (DD, J = 7.6, 5.6Hz, 1H), 2.76-2.61 (m, 2H), 2.34 (S, 3H) 2.22-1.98 (m, 2H), 1.66 (BRS, 1H); 13 C NMR (CDCL 3 100 MHz) δ (PPM): 141.78, 138.87, 137.43, 135.39, 129.32, 129.20, 128.46, 126.06, 73.80, 40.60, 31.78, 21.25, 21.13; HRMS (ESI) M / Z: C 16 Hide 18 O [m + na] + Theoretical value 249.1249, measured value 249.1243.
Embodiment 2
[0023] In the present embodiment, 1-phenyl ethanol in Example 1 was replaced with equimola 1- (2-methylphenyl) ethanol, and the other steps were the same as in Example 1, to obtain a white solid of a structural formula:
[0024]
[0025] The yield of the white solid is 52%, and the high performance liquid chromatography has an EE value of 93%, and the spectrum data is: 1 H NMR (CDCL 3 400MHz) δ (PPM): 7.53 (D, J = 8.0 Hz, 1H), 7.30-7.18 (m, 3H), 7.15 (S, 4H), 4.97 (DD, J = 8.2, 4.6 Hz, 1h), 2.89-2.82 (m, 1H), 2.78-2.70 (m, 1H), 2.37 (S, 3H), 2.79 (S, 3H), 2.79 (M, 2H), 1.79 (BRS, 1H); 13 C NMR (CDCL 3 100 MHz) δ (PPM): 142.9, 138.8, 135.3, 134.5, 130.4, 129.1, 128.4, 127.2, 126.3, 125.2, 69.9, 39.6, 31.8, 21.1, 19.0; HRMS (ESI) M / Z: C 17 Hide 20 O [m + na] + Theoretical value 263.1406, the measured value 263.1404.
Embodiment 3
[0027] In the present embodiment, 1-phenyl ethanol in Example 1 was replaced in an equimola 1- (2,4-dimethylphenyl) ethanol, and the reaction was reacted for 8 hours, and the other steps were the same as in Example 1. White solids as follows:
[0028]
[0029] The yield of the white solid is 75%, and the EE value of the EE is 92%, and the spectrum data is: 1 H NMR (CDCL 3 400MHz) δ (PPM): 7.39 (D, J = 7.6Hz, 1H), 7.12 (S, 4H), 7.06 (D, J = 8.0 Hz, 1H), 6.98 (S, 1H), 4.91 (DD, J = 8.0, 4.6Hz, 1H), 2.85-2.74 (m, 1H), 2.72-2.66 (m, 1H), 2.35 (S, 3H), 2.25 (S, 3H), 2.09 (S, 3H), 2.09 -1.99 (m, 2H), 1.79 (BRS, 1H); 13 C NMR (CDCL 3 100 MHz) δ (PPM): 139.9, 138.9, 136.9, 135.4, 134.6, 131.3, 129.2, 128.4, 127.1, 125.3, 70.1, 39.6, 32.0, 21.1, 39.0; HRMS (ESI) m / z: c 18 Hide 22 O [m + na] + Theoretical value 277.1562, measured value 277.1561.
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com