Phenolic hydroxyl resin and resist material
A technology of phenolic hydroxyl resin and novolac resin, which is applied in the field of resist materials and phenolic hydroxyl resins, can solve the problems of unusable, heat resistance, insufficient developability, inability to meet market requirements, etc., and achieve crack resistance Excellent performance and excellent alkali developability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0150] Hereinafter, the present invention will be described in more detail with reference to specific examples. In addition, the number average molecular weight (Mn), weight average molecular weight (Mw), and polydispersity coefficient (Mw / Mn) of the synthetic|combined resin are the values measured on the following GPC measurement conditions.
[0151] [Measurement conditions of GPC]
[0152] Measuring device: "HLC-8220GPC" manufactured by Tosoh Corporation
[0153] Column: Showa Denko "Shodex KF802" (8.0mmФ×300mm) + Showa Denko "Shodex KF802" (8.0mmФ×300mm)
[0154] + "ShodexKF803" made by Showa Denko Co., Ltd. (8.0mmФ×300mm) + "ShodexKF804" made by Showa Denko Co., Ltd. (8.0mmФ×300mm)
[0155] Column temperature: 40°C
[0156] Detector: RI (differential refractometer)
[0157] Data processing: "GPC-8020model II version 4.30" manufactured by Tosoh Corporation
[0158] Developing solvent: tetrahydrofuran
[0159] Flow rate: 1.0mL / min
[0160] Sample: A product obtained...
manufacture example 1 3
[0178] Production Example 1 Production of triarylmethane type compound (A-1)
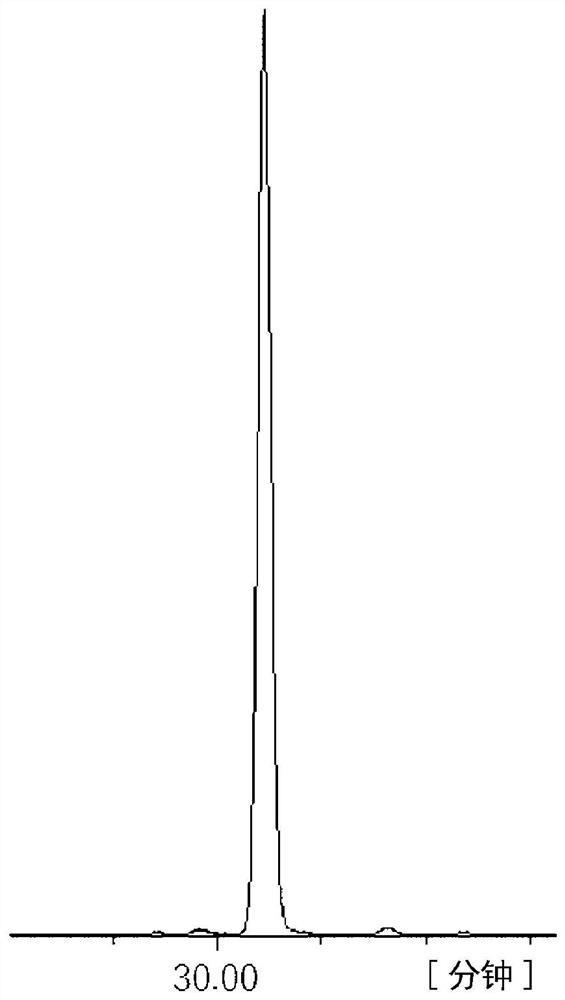
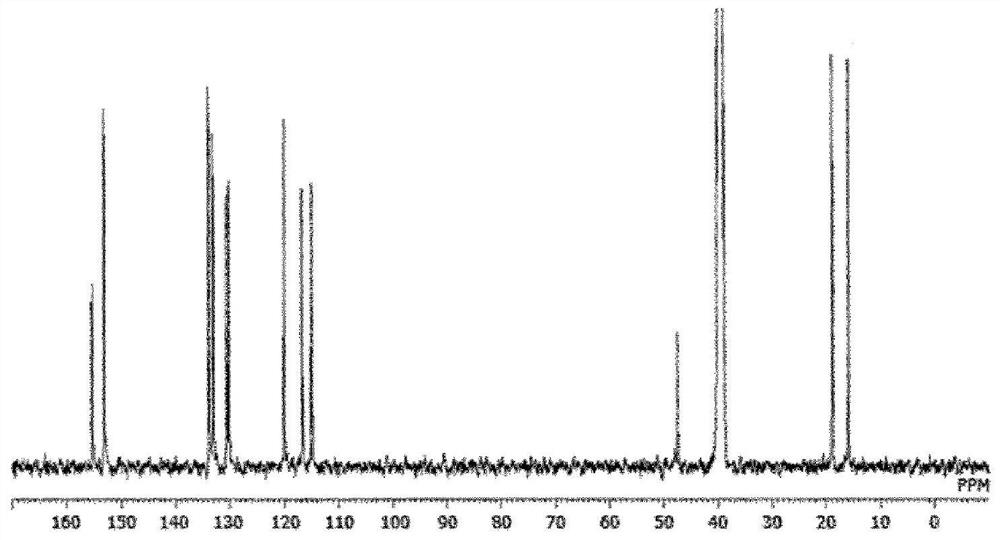
[0179] 586.4 g of 2,5-xylenol and 244 g of 4-hydroxybenzaldehyde were put into a 3000 ml 4-necked flask equipped with a condenser, and dissolved in 1000 ml of 2-ethoxyethanol. After cooling in an ice bath and adding 30 ml of sulfuric acid, the mixture was heated to 100° C. with a mantle heater and reacted while stirring for 2 hours. After the reaction, water was added to the obtained solution to reprecipitate the crude product. The crude product was redissolved in acetone and reprecipitated with water, and the precipitate was filtered and vacuum-dried to obtain 421 g of a white crystal triarylmethane compound (A-1). pass 13 C-NMR confirmed the production of a compound represented by the following structural formula. The purity calculated from the GPC chart was a GPC purity of 98.2%. The GPC chart of triarylmethane type compound (A-1) is shown in figure 1 ,Will 13 The C-NMR diagram is shown in...
manufacture example 2
[0181] Production Example 2 Production of Novolak Resin Intermediate (M-1)
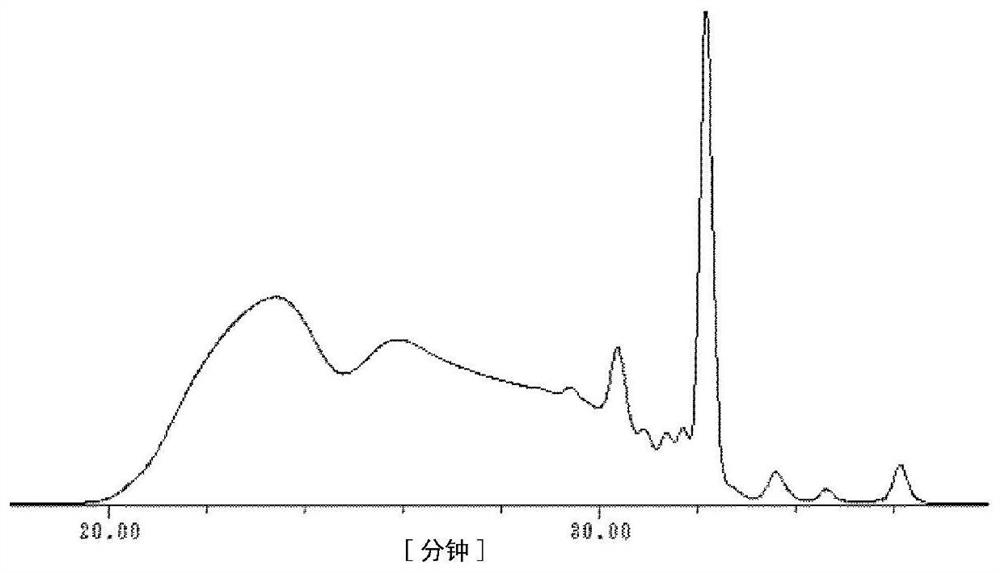
[0182] 174 g of a triarylmethane type compound (A-1) and 54 g of m-cresol were charged into a 3000 ml 4-necked flask equipped with a condenser, and then dissolved in 500 ml of 2-ethoxyethanol and 500 ml of acetic acid. After cooling in an ice bath and adding 50 ml of sulfuric acid, 33 g of 92% paraformaldehyde was added. The mixture was heated to 80° C. in an oil bath, and reacted while stirring for 10 hours. After the reaction, water was added to the obtained solution to reprecipitate the crude product. After redissolving the crude product in acetone and reprecipitating with water, the precipitate was filtered and vacuum-dried to obtain 213 g of a novolac resin intermediate (M-1) as a red powder. The number average molecular weight (Mn) of the novolac resin intermediate (M-1) was 1,937, the weight average molecular weight (Mw) was 12,822, and the polydispersity coefficient (Mw / Mn) was 6.62.
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com