A preparation method and application method of a highly dispersed copper-zinc catalyst for carbon dioxide reduction
A carbon dioxide, highly dispersed technology, applied in the direction of catalyst activation/preparation, carbon monoxide, physical/chemical process catalysts, etc., can solve the problems of poor thermal stability of catalysts, reduction of catalytic activity, deactivation, etc., to improve catalytic performance and catalytic performance Enhancement and anti-agglomeration effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
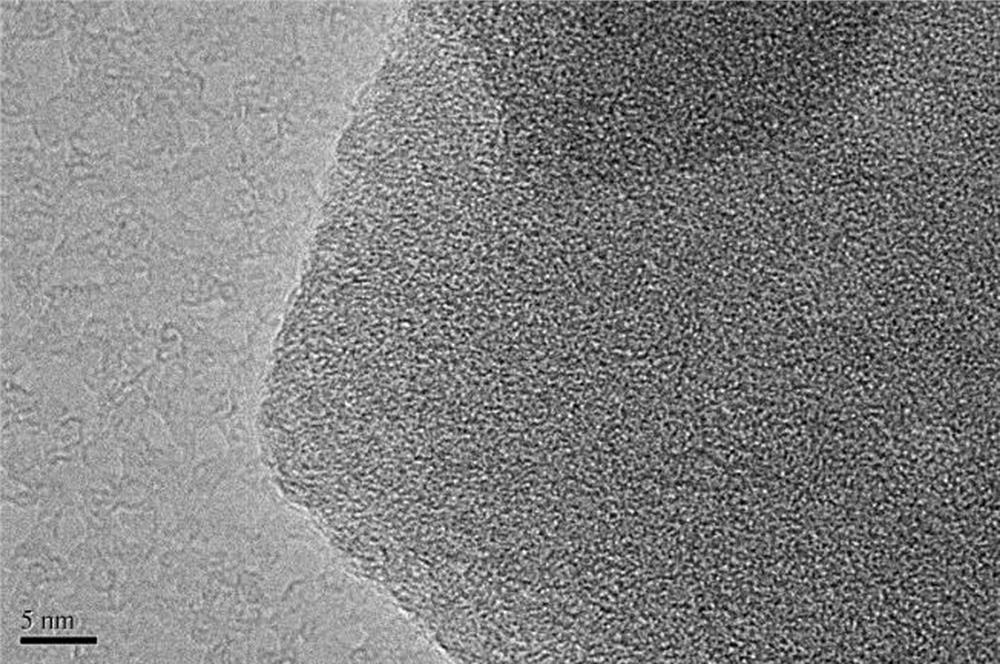
Image
Examples
Embodiment 1
[0039] (1) Prepare an ethylene glycol solution with a mass fraction of 10% copper nitrate, weigh 100 g of the solution, add 2 g of water, stir at room temperature for 1 hour, then add 2 g of acetic acid, and continue stirring for 1 hour at room temperature to obtain mixed solution A;
[0040] (2) Transfer the mixed solution A to a hydrothermal reaction kettle, and react at a constant temperature at 180 °C for 6 hours, then take out the reaction kettle, cool to room temperature, and obtain sample B;
[0041] (3) Prepare a zinc nitrate methanol solution with a mass fraction of 5%, weigh 200 g of the solution, add 50 g of sample B to it, and stir at room temperature for 30 minutes to obtain sample C;
[0042] (4) Prepare 2-methylimidazole methanol solution with a mass fraction of 5%, mix 600 g with sample C, stir at room temperature for 6 hours, centrifuge and wash, dry at 80 °C for 4 hours, and then Dry at 120°C for 4 hours to obtain sample D;
[0043] (5) Under the flowing ni...
Embodiment 2
[0045] (1) Prepare a glycerin solution of copper nitrate with a mass fraction of 10%, weigh 150 g of the solution, add 4 g of water, stir at room temperature for 1 hour, then add 4 g of acetic acid, and continue stirring for 1 hour at room temperature to obtain a mixed solution A;
[0046] (2) Transfer the mixed solution A to a hydrothermal reactor and react at a constant temperature of 160 °C for 12 hours, then take out the reactor and cool it to room temperature to obtain sample B;
[0047] (3) Prepare a zinc chloride methanol solution with a mass fraction of 5%, weigh 200 g of the solution, add 80 g of sample B to it, and stir at room temperature for 30 minutes to obtain sample C;
[0048] (4) Prepare 2-methylimidazole methanol solution with a mass fraction of 5%, mix 800 g with sample C, stir at room temperature for 12 hours, centrifuge and wash, dry at 80 °C for 4 hours, and then Dry at 120°C for 4 hours to obtain sample D;
[0049] (5) Under the flowing nitrogen gas at...
Embodiment 3
[0051] (1) Prepare an isopropanol solution of copper nitrate with a mass fraction of 10%, weigh 100 g of the solution, add 2 g of water, stir at room temperature for 1 hour, then add 2 g of acetic acid, and continue stirring for 1 hour at room temperature to obtain mixed solution A;
[0052] (2) Transfer the mixed solution A to a hydrothermal reaction kettle, and react at a constant temperature of 180 °C for 12 hours, then take out the reaction kettle, cool to room temperature, and obtain sample B;
[0053] (3) Prepare a zinc acetate methanol solution with a mass fraction of 5%, weigh 200 g of the solution, add 50 g of sample B to it, and stir at room temperature for 30 minutes to obtain sample C;
[0054] (4) Prepare 2-methylimidazole methanol solution with a mass fraction of 5%, mix 400 g with sample C, stir at room temperature for 6 hours, centrifuge and wash, dry at 80 °C for 4 hours, and then Dry at 120°C for 4 hours to obtain sample D;
[0055] (5) Under the flowing ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com