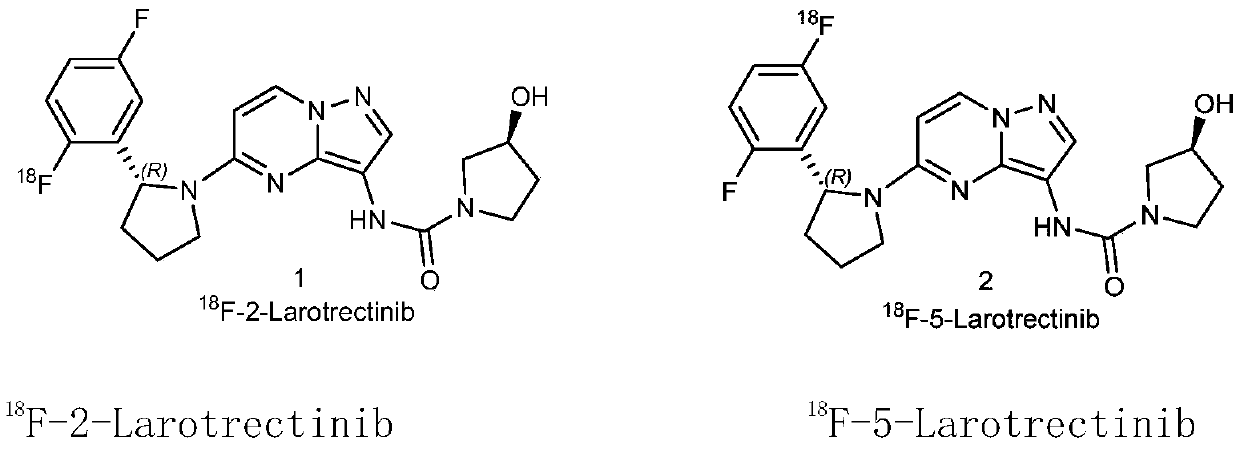
Radioactive fluorine labeled Larotrectinib compound and preparation method thereof
A compound and labeling technology, which can be used in radioactive carriers, chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, etc., and can solve problems such as no more effective technical means.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Esterification of benzoyl chloride to prepare I-Bz-2-Larotrectinib or I-Bz-5-Larotrectinib
[0109] In a 2000ml reaction flask, add 400mL of chloroform, compound I-2-Larotrectinib (80mmol, 42.9g), stir at room temperature until dissolved, add pyridine (240mmol, 18.98g), and simultaneously add benzoyl chloride (80mmol, 11.4g) , the mixture was stirred and reacted at room temperature for 3 hours, and controlled by TLC. After the reaction was completed, the mixture was poured into water, and the organic layer was separated, followed by dilute HCl (to remove pyridine; at the same time, let the unreacted acid chloride become acid), and then dilute NaOH (preferably saturated sodium bicarbonate, 100 mL) to remove acid, saturated sodium chloride to neutrality, and remove most of the water in the organic layer. The organic layer was dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent, and the residue was crystallized with ethanol-methyl...
Embodiment 2
[0112] Esterification of benzoyl chloride to prepare I-Bz-5-Larotrectinib
[0113] The preparation method was the same as in Example 1, and the compound I-5-Larotrectinib was used as the raw material to obtain the product I-Bz-5-Larotrectinib as a white solid with a yield of 88% (70.4 mmol, 45.1 g). The route is as follows:
[0114]
Embodiment 3
[0116] Esterification of pivaloyl chloride to prepare I-Piv-2-Larotrectinib
[0117] In a 2000 ml reaction flask, add 400 mL of chloroform, compound I-2-Larotrectinib (80 mmol, 42.9 g), stir at room temperature until dissolved, and simultaneously add saturated sodium bicarbonate solution (23 g) and pivaloyl chloride (80 mmol, 9.64 g) , the mixture was stirred and reacted at room temperature for 24 hours, and controlled by TLC. After the reaction was completed, the mixture was poured into water, and the organic layer was separated, washed with 1N sodium hydroxide (100mL), washed with saturated brine (100mL), dried over anhydrous magnesium sulfate, and reduced Concentrated under reduced pressure to remove the solvent, and the residue was crystallized from methanol-isopropyl ether (7:3, 150 mL) to obtain the product I-Piv-2-Larotrectinib as a white solid with a yield of 85% (68 mmol, 42.2 g). The route is as follows:
[0118]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com