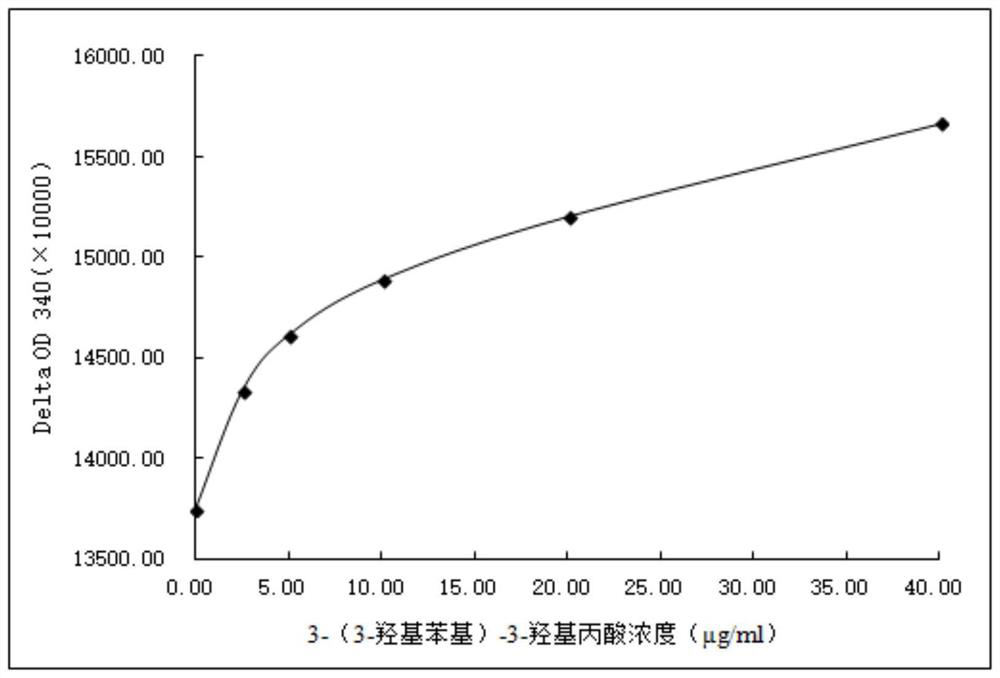
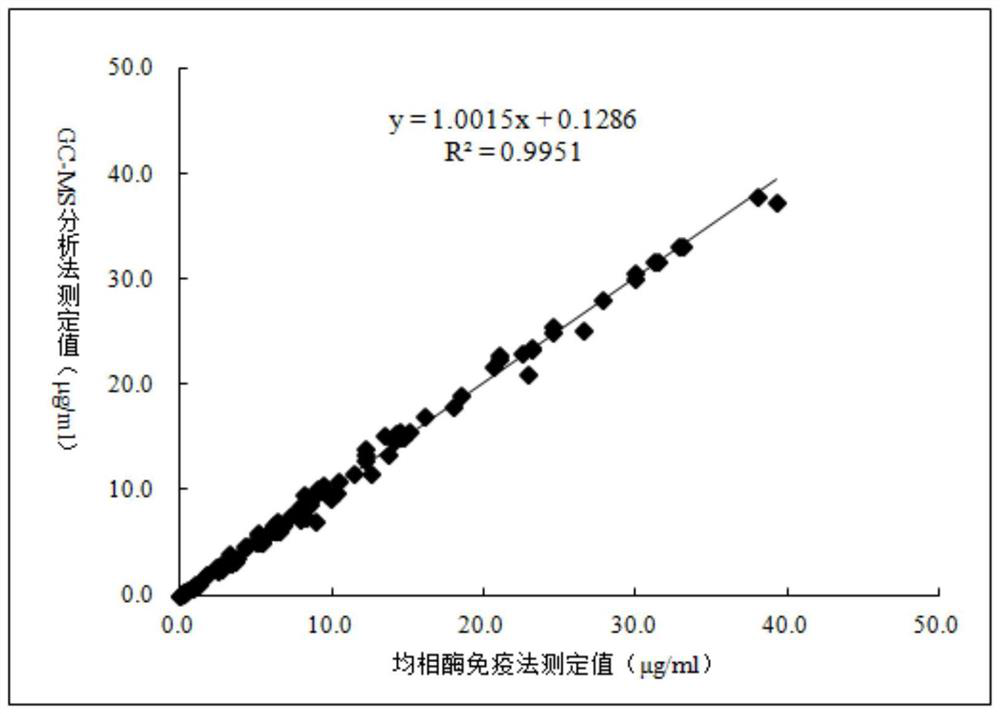
3-(3-hydroxyphenyl)-3-hydroxypropionic acid derivative, homogeneous enzyme immunoassay reagent and preparation method thereof
A technology of hydroxypropionase and homogeneous enzyme immunity, which is applied in the preparation of carboxylic acid esters, carboxylic acid esters/lactones, organic compounds, etc., can solve problems such as complicated operations, and achieve high immunogenicity and easy Realize and promote the effect of high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078]Example 1. Synthesis of 3- (3-hydroxyphenyl) -3-hydroxypropionic acid derivative
[0079]The synthesis route of 3- (3-hydroxyphenyl) -3-hydroxypropionic acid derivative is as follows:
[0080]
[0081]The preparation process of 3- (3-hydroxyphenyl) -3-hydroxypropionic acid derivative is as follows:
[0082](1) Compound 3 synthesis:
[0083]20 g of compound 1, i.e., 3- (3-hydroxyphenyl) -3-hydroxypropionic acid (purchased from Sigma) and 33.2 g of Compound 2 (purchased from Sigma), co-dissolved in 300 ml DMF, then Join 36.4 g of K2CO3After the reaction mixing solution was prepared, the reaction mixed solution was stirred at room temperature for 12 hours, and 200 ml of purified water was added to the reaction solution, and then this solution was extracted with 300 ml of DCM, and the extraction step was repeated 3 times, Extractable organic phase through NA2SO4The drying treatment was carried out, and the residue obtained after concentration was purified by the silica gel drying column (PE: EA ...
Embodiment 2
[0086]Example 2. Synthesis of 3- (3-hydroxyphenyl) -3-hydroxypropionic acid immunogen
[0087]The 3- (3-hydroxyphenyl) -3-hydroxypropionic acid immunization in this embodiment is formed by 3- (3-hydroxyphenyl) -3-hydroxypropionic acid derivatives obtained from the BSA. The structural formula is as follows (II):
[0088]
[0089]Where the carrier is BSA.
[0090]The synthesis method of the 3- (3-hydroxyphenyl) -3-hydroxypropionic immunogen is as follows:
[0091](1) Weigh 2.72 g of dihydrogen phosphate, 4.26 g of sodium hydrogen phosphate, 8.5 g of sodium chloride, 0.95 g of magnesium chloride, and dissolve in 1 L of deionized water, adjusting pH to 8.2, making buffer A;
[0092](2) Those 3 ml of BSA, -4 ° C were weeded in 3 ml of buffer A, and a 1 mg / ml vector solution was made;
[0093](3) Weigh 3- (3-hydroxyphenyl) -3-hydroxypropionic acid derivative obtained by 3 mg Example 1, dissolved in 300 μl of buffer A at -4 ° C, 3- for 10 mg / ml of 3- (3-hydroxyphenyl) -3-hydroxypropionic acid derivative so...
Embodiment 3
[0096]Example 3. Synthesis of 3- (3-hydroxyphenyl) -3-hydroxypropionic acid immunogen
[0097]The 3- (3-hydroxyphenyl) -3-hydroxypropionic acid immunization in this example is 3- (3-hydroxyphenyl) -3-hydroxypropionic acid derivatives obtained from the oval protein. Cheng, its structural formula is as follows (II)
[0098]
[0099]Where the carrier is a folin protein.
[0100]The synthesis method of the 3- (3-hydroxyphenyl) -3-hydroxypropionic immunogen is as follows:
[0101](1) Weigh 2.3 g of pelvic phosphate, 3.8 g of sodium hydrogen phosphate, 8.0 g of sodium chloride, 0.7 g of magnesium chloride, and dissolve in 0.9L deionized water, adjust the pH to 8.0, and make buffer A;
[0102](2) Weigh 6 mg of ovantin, dissolved in 3 ml of buffer A at -4 ° C, and formulated a 2 mg / ml vector solution;
[0103](3) Weigh 2.4 mg Example 1 obtained from 3- (3-hydroxyphenyl) -3-hydroxyacionic acid derivatives, -4 ° C dissolved in 300 μL of the buffer A, 300 mg / ml 3 - (3-hydroxyphenyl) -3-hydroxypropionic acid de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com