Fluorescence immunosorbent assay kit for joint detection of SAA (Serum Amyloid A) and CRP (C-reactive protein) based on two-color quantum dots and preparation method of fluorescence immunosorbent assay kit
A detection kit and fluorescence immunology technology, applied in biological testing, material inspection products, etc., can solve the problems of further improvement of stability and sensitivity, and high detection cost of chemiluminescence detection kits, so as to reduce technical difficulty and operational errors, Stable and accurate detection results and stable fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] In the present invention, the polymer encapsulation method is preferably described by taking polymaleic anhydride cetyl ester modified CdSe / ZnS quantum dots as an example. The preparation method of described poly-cetyl maleic anhydride modified CdSe / ZnS quantum dots, preferably comprises the following steps:
[0044] ①CdSe / ZnS quantum dots are mixed with chloroform and ultrasonicated to obtain a dispersion;
[0045] ② polycetyl maleic anhydride and chloroform are mixed, and ultrasonicated to obtain a polycetyl maleic anhydride chloroform solution;
[0046] ③ mixing the dispersion with the polycetyl maleic anhydride chloroform solution, ultrasonically removing the chloroform to obtain the poly cetyl maleic anhydride-CdSe / ZnS conjugate;
[0047] ④ Mix the polycetyl maleic anhydride-CdSe / ZnS conjugate, water and ammonia water, stir, and separate the solid and liquid to obtain the polycetyl maleic anhydride modified CdSe / ZnS quantum dots.
[0048] In the present invention...
Embodiment 1
[0081] Composition and preparation of the kit
[0082] (1) Composition of the kit
[0083] Coating antibody 1: CRP monoclonal antibody, purchased from Beijing Baichuan Feihong Biotechnology Co., Ltd., the product catalog number is 7D9, and the concentration is 2.2 mg / mL. SAA monoclonal well antibody was purchased from Shanghai Unionwell Biotechnology Co., Ltd., the product catalog number is 2201, and the concentration is 5.0 mg / mL.
[0084] Conjugated antibody 2: CRP monoclonal antibody, purchased from Hangzhou Qitai Co., Ltd., the product catalog number is MC02, and the concentration is 5 mg / mL. SAA monoclonal well antibody was purchased from Shanghai Unionwell Biotechnology Co., Ltd., the product catalog number is 2203, and the concentration is 5.1 mg / mL.
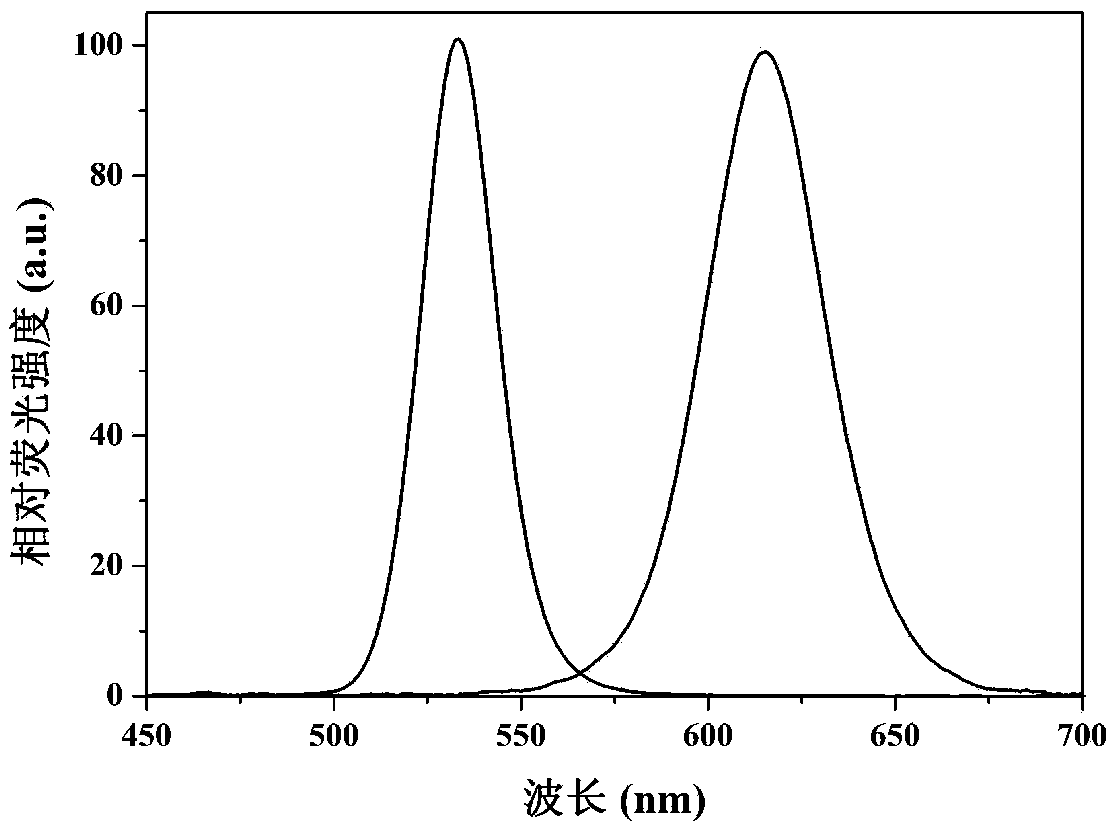
[0085] Fluorescence-labeled antibody 2: green quantum dot-bound CRP monoclonal well antibody, red quantum dot-bound SAA monoclonal well antibody.
[0086] Standard products: CRP standard products were purchased from Ha...
Embodiment 2
[0099] Detection method
[0100] (1) Preparation of standard curve
[0101] (1) Incubation of standard products: Take out the microplate and return to room temperature. Dilute the CRP and SAA standards with sample diluent to the target concentration, for example, the concentration of CRP standard solution: 0, 5, 10, 25, 50, 100, 200, 400, 800, 1000ng / mL; the concentration of SAA standard Solution: 0, 5, 10, 25, 50, 100, 200, 400, 800, 1000 ng / mL. After mixing, add 100 μL to each well, place in a constant temperature and humidity incubator, and incubate at 37°C for 30 minutes; shake off the reaction solution, wash with washing solution five times, and pat dry on absorbent paper.
[0102] (2) Incubation of labeled antibody: dilute SAA antibody 2 with antibody 2 diluent, dilute 1:100 times, 100 μL per well, put it in a constant temperature and humidity incubator, and incubate at 37°C for 30 minutes. Shake off the reaction solution, wash five times with washing solution, and pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Coating concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com