Chimeric enzyme antibiotic for killing staphylococcus aureus and preparation and application thereof
A technique for staphylococcus and staphylococcus infection, applied in biochemical equipment and methods, preparations for in vivo tests, medical preparations containing active ingredients, etc., can solve problems such as no good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 designs primers, PCR amplifies target gene fragment
[0048] Referring to the genome sequences of Staphylococcus aureus phage, cytoplasmic transduction peptide and green fluorescent protein GFP in GenBank, the lyase JDlys gene, three transmembrane peptides (CTP Tat 、CTP ANT and CTP TP10 ) gene and green fluorescent protein GFP gene; design primers, use the synthesized gene as a template, and use PCR technology to amplify the target gene, wherein the size of JDlys gene is 1488bp, CTP Tat The gene size is 33bp, CTP ANT Gene size is 48bp, CTP TP10 The gene size is 63bp, and the GFP gene size is 720bp. The specific operation is as follows:
[0049] Primers were designed, and the primer sequences are listed in Table 1. Using the DNA of synthetic lyase JDlys gene, transmembrane peptide CTP gene and green fluorescent protein GFP as templates, the JDlys gene fragment was amplified with primers JDlys-F1 / JDlys-R; primers CTP Tat -F / CTP Tat -R, CTP Ant -F / C...
Embodiment 2
[0052] Example 2 Construction, expression and purification of recombinant plasmid pET-28a(+)-CTP-JDlys
[0053] Using the prokaryotic expression system pET-28a(+), the JDlys gene product obtained in Example 1 was used to construct a recombinant plasmid to obtain the recombinant plasmid pET-28a-JDLys, and then the 5' end of the JDlys gene in pET-28a-JDlys was digested, Insert CTPs or GFP genes, respectively. Sequence analysis and comparison were then carried out to determine the recombinant plasmid pET-28a-CTP with the correct sequence Tat -JDlys, pET-28a-CTP Ant -JDlys, pET-28a-CTP TP10 -JDlys and pET-28a-GFP-CTP Tat -JDlys. Transform the above-mentioned recombinant plasmid into the expression recipient bacteria-Escherichia coli BL21(DE3), screen positive clones for induced expression and identification of the protein, and obtain recombinant JDlys protein and chimeric lyase recombinant protein CTP Tat -JDlys, CTP Ant -JDlys, CTP TP10 -JDlys and GFP-CTP Tat -JDlys, th...
Embodiment 3
[0056] Embodiment 3 Recombinant lyase in vitro minimum inhibitory concentration (MIC) determination
[0057] The recombinant expressed protein obtained in Example 2 was subjected to an in vitro lysis test to check the lysis activity and lysis spectrum. The specific operation is as follows:
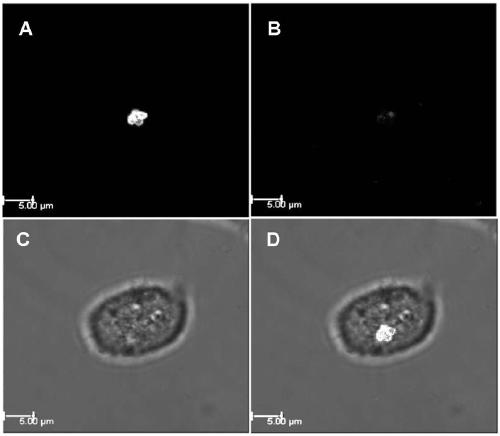
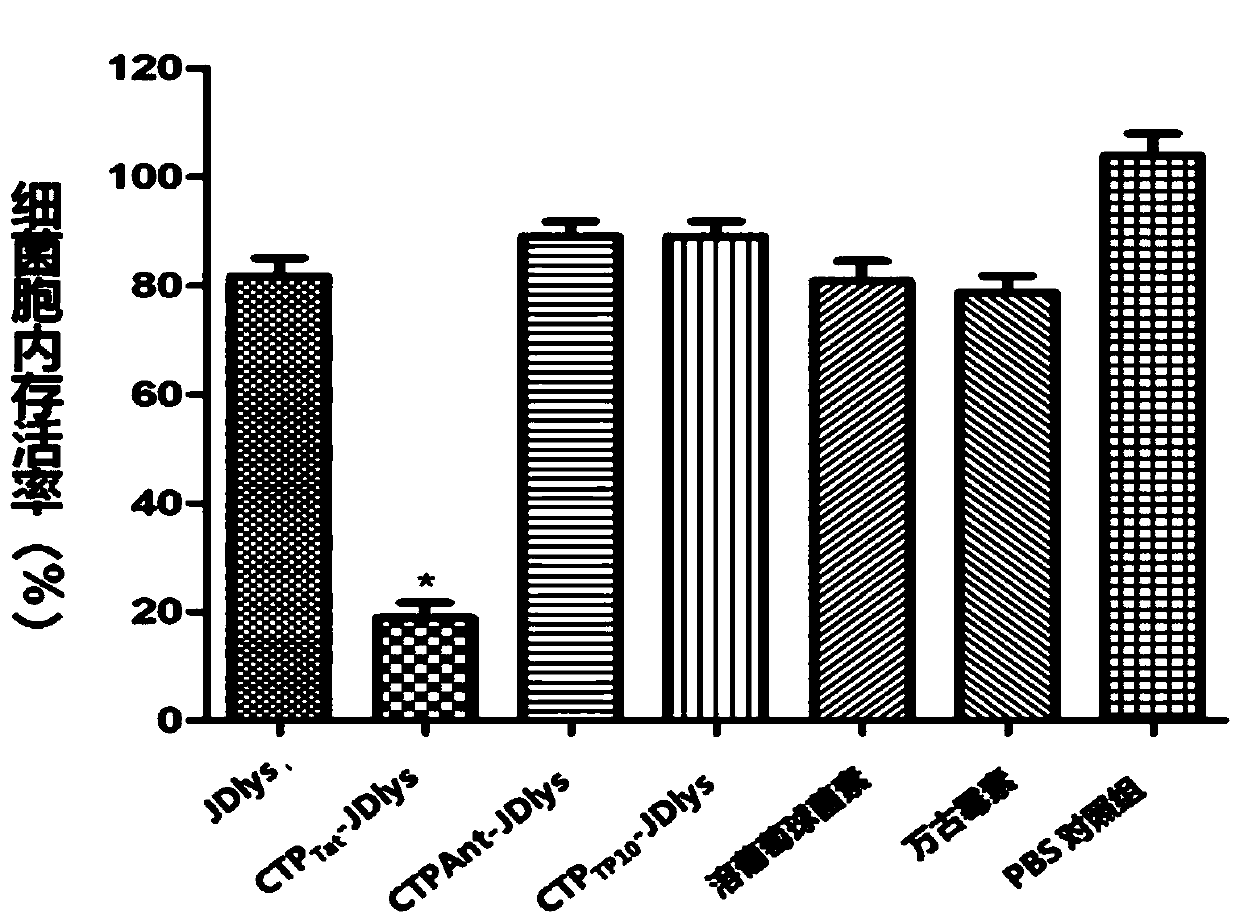
[0058] Staphylococcus strains of different origins were grown to early logarithmic phase (OD600 of 0.2 to 0.4). After washing three times with PBS, take 100 μl of bacterial suspension (diluted to 5×10 6 cfu / mL) was mixed with 100 μl of recombinant lyase at different final concentrations (10, 20, 40, 80, 160, 320 and 640 μg / mL), and the MIC of the lyase was determined in a 96-well microtiter plate. Plates were incubated at 37°C for 20 hours and read with a 96-well plate reader (OD 600 ).
[0059] The experimental results are shown in Table 2, Tat type chimeric recombinant lyase CTP Tat -JDlys showed a slightly reduced bactericidal effect on a small number of strains compared with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com