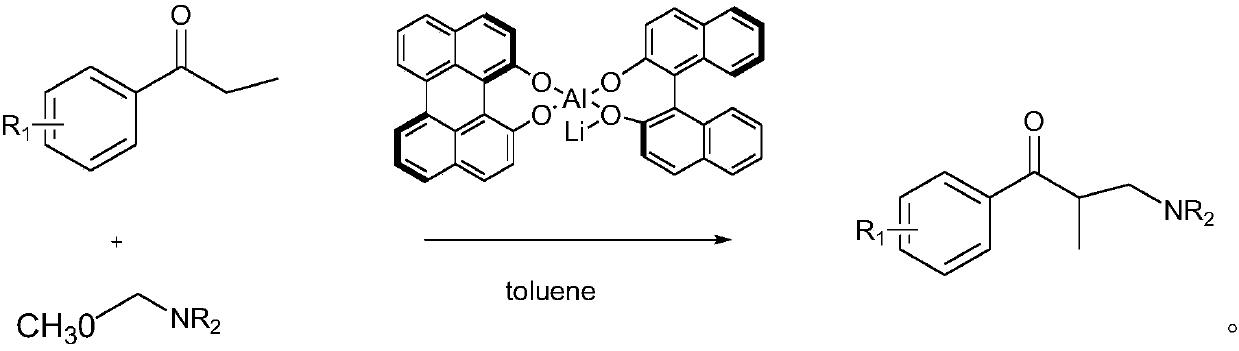
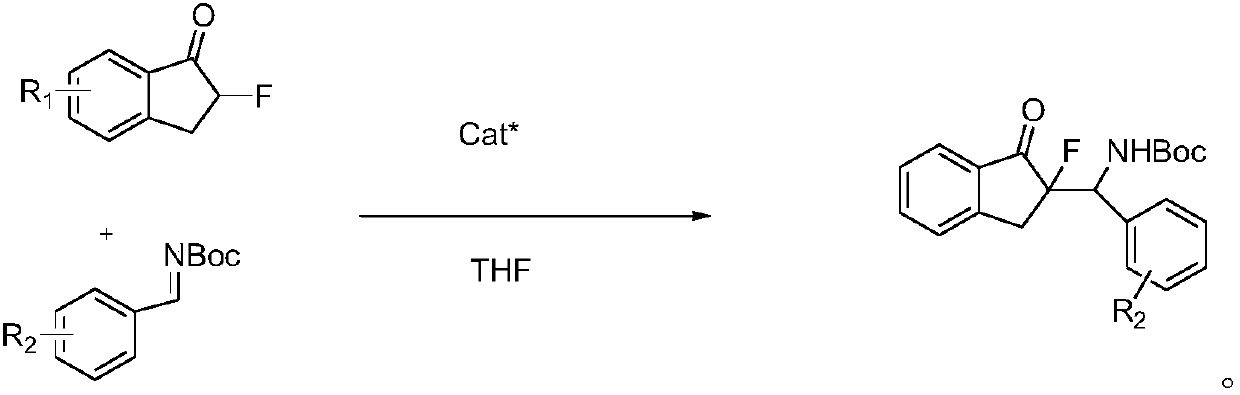
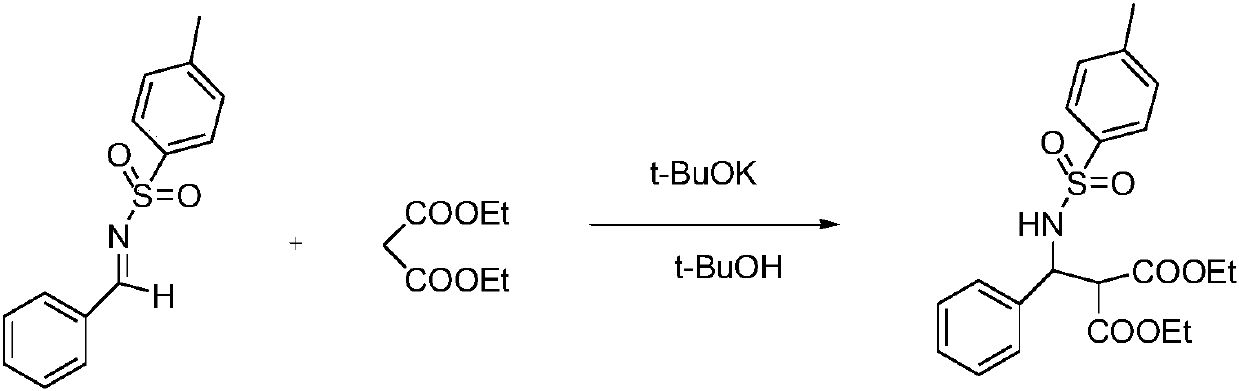
Chiral amino sulfamide ligand metal complexes and applications in catalytic reactions
A technology of aminosulfonamide and sulfonimide, which is applied to chiral aminosulfonamide ligand metal complex and its application in catalytic reaction, can solve the problem that the product does not have chirality, the substrate universality is poor, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 compound (L-1)
[0055] Dissolve unilateral protection one, diphenylethylenediamine (4mmol) in ethanol (50mL), slowly add phenol dialdehyde (1.8mmol), react at room temperature for 12h, then add NaBH at 0°C 4 (14.4mmol), after the addition was complete, react at room temperature and monitor by TLC. After the reaction was complete, water (120mL) was added to the reaction solution, and 2 Cl 2 extraction. The organic phase was collected, washed with saturated brine and dried over anhydrous sodium sulfate; concentrated under reduced pressure, separated and purified by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the ligand compound (L-1).
[0056] The synthetic route of compound (L-1) is as follows:
[0057]
[0058] Referring to Example 1, compounds L1-L8 were prepared. The specific structural analysis data and physical and chemical parameters of L1-L8 are as follows.
[0059] L1: white solid; [α] D 25 =-...
Embodiment 2
[0067] The preparation of embodiment 2 formula (I-1)
[0068] The chiral sulfamoamide ligands (L1-L8) prepared in the above examples were used to prepare the formula (I-1) according to the following route and operation steps. The specific route is:
[0069]
[0070] ; The specific operation is: add nickel acetate (0.02mmol), chiral aminosulfonamide ligand (0.01mmol), tetrahydrofuran (4mL) and a stir bar into the reaction vessel, and stir at 35°C for 1.5h.
[0071] Ar is p-nitrophenyl (4-NO 2 -C 6 h 4 ) as an example, that is, the chiral aminosulfonamide ligand L4 as an example, the molecular weight of the complex structural formula is obtained through simulation to be 1157.15115, while the molecular weight obtained in the actual characterization test is 1157.15010, which is basically consistent. It can be seen that the structure of the complex is inferred that the ratio of chiral sulfamoamide ligand L to nickel acetate is 1:2.
Embodiment 3
[0075] A metal complex formed by chiral sulfamoamide ligand L and nickel acetate catalyzes the direct Mannich reaction of benzenesulfonimide and diethyl malonate. .
[0076] Concrete reaction route is as follows:
[0077]
[0078] ; Specific operation steps: Add nickel acetate (0.02mmol), chiral sulfamoamide ligand L (0.01mmol), tetrahydrofuran (4mL) and a stirrer into a reaction vessel, and stir at 35°C for 1.5h. Then add benzenesulfonimide 1a (1mmol), tetrahydrofuran (0.5mL), stir at 0°C for 0.5h, then add diethyl malonate (1mmol) and tetrahydrofuran (0.5mL), stir at 0°C for 16h -17h, TLC detection reaction. Separation and purification by column chromatography, the enantiomeric excess of the product was determined by high performance liquid chromatography (Daicel chiralcel IA, V 正己烷 :V 异丙醇 =70:30, flow rate 1.0mL / min) to collect the target compound 2a. [α] 25 D =+29.6 (c=0.69in CH 2 Cl 2 ); 1 H NMR (500MHz, CDCl 3 )δ7.49(d, J=7.70Hz, 2H), 7.10(s, 5H), 7.04(d, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com