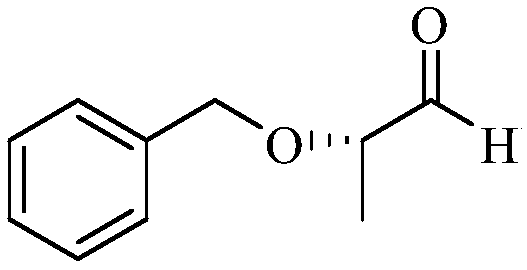
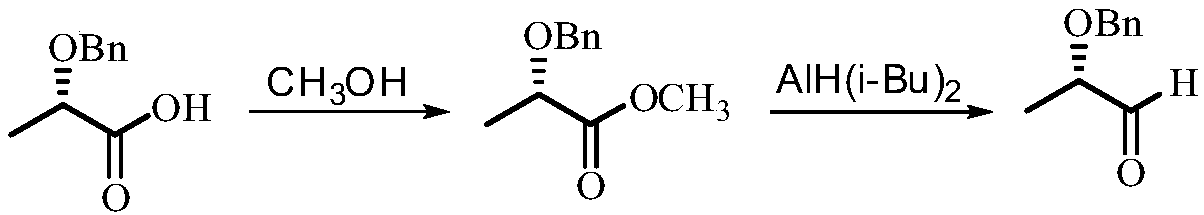
Synthetic method of (S)-2-benzyloxy propionaldehyde
A technology for the synthesis of benzyloxypropanal and its synthesis method, which is applied in the field of synthesis of -2-benzyloxypropanal, can solve the problems of unfavorable large-scale production, low atom economy, high production risk, etc., and achieve improved operation Safety, ease of industrial production, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Add (S)-2-benzyloxypropionic acid (18.02 grams, 0.10mol), thionyl chloride (14.16 grams, 0.12mol) and 150ml toluene in a 250ml round bottom flask, heat to reflux, and use sodium hydroxide for the tail gas The solution absorbs. After 6 hours of reaction, the heating was stopped, and the toluene and excess thionyl chloride were removed by rotary evaporation to obtain 18.87 g (0.095 mol) of (S)-2-benzyloxypropionyl chloride, with a yield of 95%.
[0031] 2. 10.00 grams of palladium barium sulfate catalyst (converted into metal palladium 0.01mol) with a palladium content of 10% is added in 100ml o-xylene, and the hydrogen atmosphere (0.1MPa pressure) is refluxed for 20 minutes and then down to room temperature; (1) 19.86 grams (0.10mol) of (S)-2-benzyloxypropionyl chloride prepared, then continue hydrogenation under 0.1MPa hydrogen atmosphere and reflux until the reaction mixture does not absorb hydrogen (hydrogen pressure does not drop in the reaction system ). After ...
Embodiment 2
[0036] 1. Add (S)-2-benzyloxypropionic acid (18.02 grams, 0.10mol), oxalyl chloride (12.70 grams, 0.10mol) and 150ml toluene in a 250ml round bottom flask, heat to reflux, and use sodium hydroxide solution for the tail gas absorb. After 8 hours of reaction, the heating was stopped, and the toluene and excess oxalyl chloride were removed by rotary evaporation to obtain 18.27 g (0.092 mol) of (S)-2-benzyloxypropionyl chloride, with a yield of 92%.
[0037] 2. Add 10.00 grams of palladium barium sulfate catalyst with a palladium content of 10% (converted to 0.01 mol of metal palladium) into 100 ml of toluene, reflux under hydrogen atmosphere (0.1 MPa) for 20 minutes and then cool down to room temperature, add (S)-2 - 19.86 grams (0.10 mol) of benzyloxy propionyl chloride, then continue hydrogenation under 0.1 MPa hydrogen atmosphere and reflux until the reaction mixture does not absorb hydrogen (hydrogen pressure does not drop in the reaction system). After the reaction, the mix...
Embodiment 3
[0040] 1. Add (S)-2-benzyloxypropionic acid (18.02 g, 0.10 mol), phosphorus trichloride (15.07 g, 0.11 mol) and 150 ml of toluene into a 250 ml round bottom flask, heat to reflux, and oxidize the tail gas with hydrogen Sodium solution absorbed. After 7 hours of reaction, the heating was stopped, and the toluene and excess thionyl chloride were removed by rotary evaporation to obtain 19.06 g (0.096 mol) of (S)-2-benzyloxypropionyl chloride, with a yield of 96%.
[0041]2. Add 12 grams of palladium-barium sulfate catalyst with a palladium content of 10% (converted to 0.012 mol of metal palladium) into 100ml of toluene, reflux under hydrogen atmosphere (0.1MPa) for 20 minutes and then cool down to room temperature, add (S)-2 - 21.41 grams (0.108 mol) of benzyloxy propionyl chloride, then continue hydrogenation under 0.1 MPa hydrogen atmosphere and reflux until the reaction mixture does not absorb hydrogen (the hydrogen pressure in the reaction system does not drop). After the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com