Hemostasis material for clinical internal medicine to stop bleeding and preparation method thereof
A hemostatic material, a technique of internal medicine, applied in medical science, surgical adhesives, bandages, etc., can solve problems such as difficulty in ensuring compatibility, absorption, easy adhesion and remaining in tissue blood vessels, increasing blood concentration and viscosity, etc. To achieve the effect of promoting wound tissue healing and coagulation, excellent biocompatibility, and promoting dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
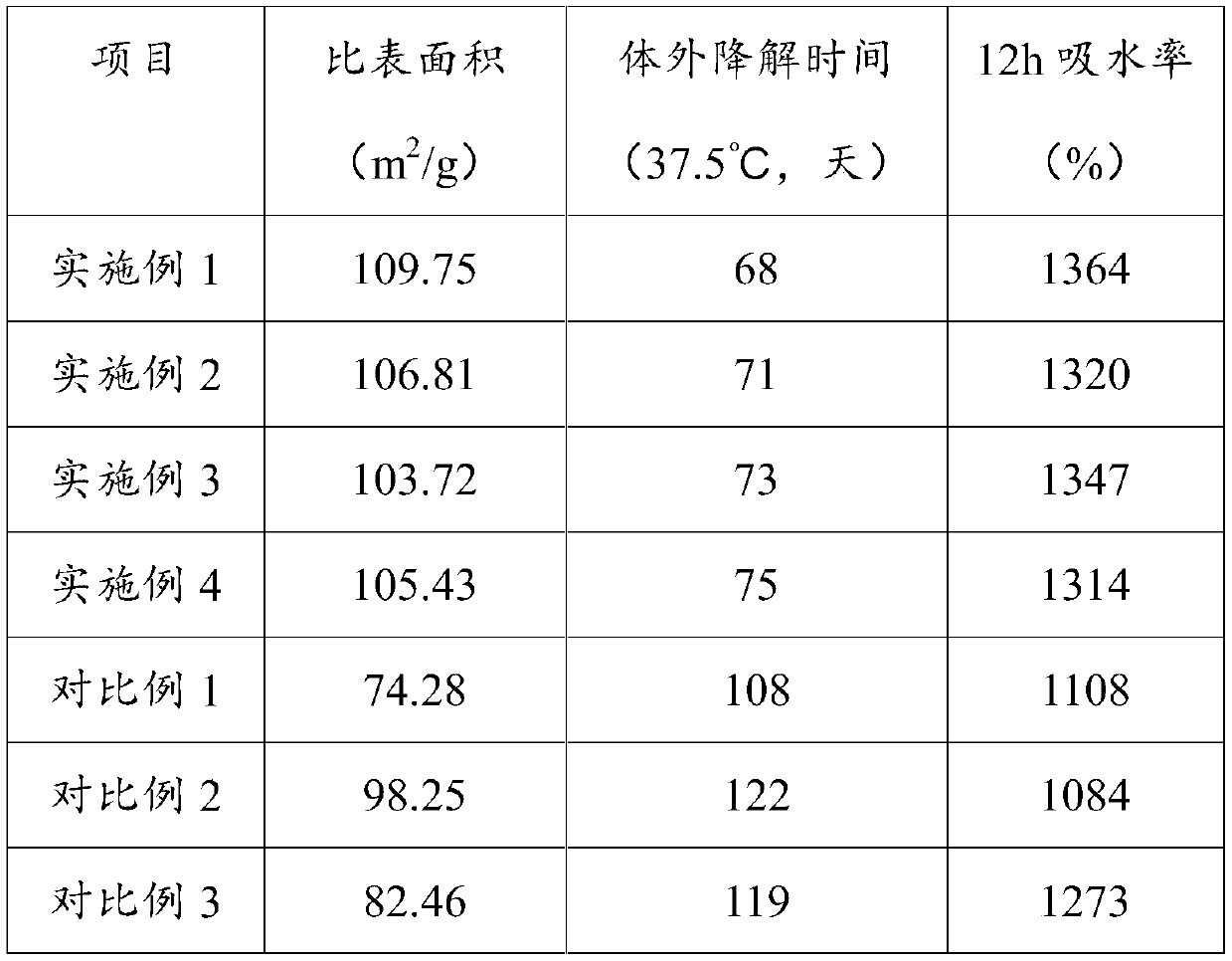
Embodiment 1
[0037] The hemostatic material for clinical hemostasis in this embodiment is prepared by loading medical glue on modified chitosan fibers, and then through the process of rapid cooling at low temperature-radiation cross-linking-freeze-drying. The medical glue is prepared by stirring and mixing methyl cyanoacrylate and polylactic acid at a mass ratio of 7:1.
[0038] Wherein, the preparation method of modified chitosan fiber comprises the following steps:
[0039] 1) Softening: put the natural chitosan crude product in a three-necked flask, connect a mechanical stirrer and a thermometer, add 8-10 times the amount of deionized water, add sodium hydroxide powder under stirring until the pH is 7.2-7.5, add Acylated amino acid hydrolase with 0.1%-0.3% natural chitosan quality, heat up to 62-68°C at a rate of 3-5°C / min, keep warm and stir for 40-50min, let stand for 30-50min, together with softener Feed into a plate and frame filter press for pressure filtration, and the filtrate i...
Embodiment 2
[0049] The hemostatic material for clinical hemostasis in this embodiment is prepared by loading medical glue on modified chitosan fibers, and then through the process of rapid cooling at low temperature-radiation cross-linking-freeze-drying. The medical glue is prepared by stirring and mixing ethyl cyanoacrylate and polylactic acid at a mass ratio of 8:1.
[0050] Wherein, the preparation method of modified chitosan fiber is identical with embodiment 1. The preparation method of the softening agent is as follows: in parts by weight, 52 parts of vinyl acetate-ethylene copolymer emulsion, 15 parts of triethanolamine, 6 parts of pure chitosan, and 62 parts of deionized water are mixed and stirred evenly, and left to stand to form gel powder , placed in a reaction kettle at 55°C, stirred at a speed of 260r / min for 35min, passed through high-temperature airflow and dried into softener powder. The natural antioxidant is vitamin E, and the antibacterial agent is chitin.
[0051] W...
Embodiment 3
[0057] The hemostatic material for clinical hemostasis in this embodiment is prepared by loading medical glue on modified chitosan fibers, and then through the process of rapid cooling at low temperature-radiation cross-linking-freeze-drying. The medical glue is prepared by stirring and mixing ethyl cyanoacrylate and polylactic acid according to the mass ratio of 7.2:1.
[0058] Wherein, the preparation method of modified chitosan fiber is identical with embodiment 1. The preparation method of the softening agent is as follows: in parts by weight, 47 parts of vinyl acetate-ethylene copolymer emulsion, 13 parts of triethanolamine, 7 parts of pure chitosan, and 63 parts of deionized water are mixed and stirred evenly, and left to stand to form gel powder , placed in a reaction kettle at 58°C, stirred at a speed of 260r / min for 40min, passed through high-temperature airflow and dried into softener powder. The natural antioxidant is catechol, and the antibacterial agent is hinoki...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com